Prediction of response to tapentadol in chronic low back pain
- PMID: 27510567
- PMCID: PMC5248647
- DOI: 10.1002/ejp.926
Prediction of response to tapentadol in chronic low back pain
Abstract
Background: Many chronic low back pain (cLBP) patients do not satisfactorily respond to treatment. The knowledge of responders and non-responders before initiating treatment would improve decision making and reduce health care costs. The aims of this exploratory prediction study in cLBP patients treated with tapentadol were to identify predictors of treatment outcome based on baseline characteristics, to evaluate quality-of-life and functionality as alternative outcome parameters and to develop nomograms to calculate the individual probability of response.
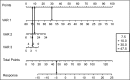
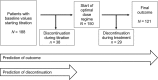
Methods: In a retrospective analysis of an open-label phase 3b trial, 46 baseline characteristics were included into statistical prediction modelling. One hundred and twenty-one patients were followed up during the titration and treatment period and 67 patients were analysed who discontinued the trial.
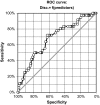
Results: Demographic data were not relevant for response prediction. Nine baseline co-variables were robust: painDETECT score, intensity of burning and painful attacks, SF36 Health Survey score (MCS, PCS), EuroQol-5, Hospital Anxiety/Depression Scale. Gender had a minor influence. Alternative outcomes (quality-of-life, functionality) were more important for response prediction than conventional pain intensity measures. Neuropathic symptoms (high painDETECT score) had a positive predictive validity. Painful attacks and classical yellow flags (depression, anxiety) negatively influenced the treatment response. High depression scores, female gender and low burning predicted discontinuation during titration.
Conclusion: In this exploratory study, predictive baseline characteristics have been identified that can be used to calculate the individual probability of tapentadol response in cLBP. The small sample size in relation to the number of initial variables is a limitation of this approach.
Significance: Predictors for treatment response of tapentadol were identified in patients with chronic low back pain based on clinical pre-treatment characteristics that can guide personalized treatment. Quality-of-life and functionality were the most relevant outcomes for response prediction.
© The Authors. European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation - EFIC®.
Figures



References
-
- Attal, N. , Perrot, S. , Fermanian, J. , Bouhassira, D. (2011). The neuropathic components of chronic low back pain: A prospective multicenter study using the DN4 Questionnaire. J Pain 12, 1080–1087. - PubMed
-
- Baron, R. , Forster, M. , Binder, A. (2012). Subgrouping of patients with neuropathic pain according to pain‐related sensory abnormalities: A first step to a stratified treatment approach. Lancet Neurol 11, 999–1005. - PubMed
-
- Baron, R. , Kern, U. , Muller, M. , Dubois, C. , Falke, D. , Steigerwald, I. (2015). Effectiveness and tolerability of a moderate dose of tapentadol prolonged release for managing severe, chronic low back pain with a neuropathic component: An Open‐label Continuation Arm of a Randomized Phase 3b Study. Pain Pract 15, 471–486. - PubMed
-
- Baron, R. , Likar, R. , Martin‐Mola, E. , Blanco, F.J. , Kennes, L. , Muller, M. , Falke, D. , Steigerwald, I. (2016). Effectiveness of Tapentadol Prolonged Release (PR) compared with Oxycodone/Naloxone PR for the Management of Severe Chronic Low Back pain with a Neuropathic Component: A randomized, controlled, Open‐Label, Phase 3b/4 Study. Pain Pract 16, 580–599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

