Evaluation of Hologic Aptima HIV-1 Quant Dx Assay on the Panther System on HIV Subtypes
- PMID: 27510829
- PMCID: PMC5035401
- DOI: 10.1128/JCM.01350-16
Evaluation of Hologic Aptima HIV-1 Quant Dx Assay on the Panther System on HIV Subtypes
Abstract
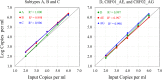
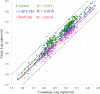
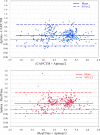
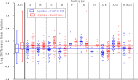
Quantitation of the HIV-1 viral load in plasma is the current standard of care for clinical monitoring of HIV-infected individuals undergoing antiretroviral therapy. This study evaluated the analytical and clinical performances of the Aptima HIV-1 Quant Dx assay (Hologic, San Diego, CA) for monitoring viral load by using 277 well-characterized subtype samples, including 171 cultured virus isolates and 106 plasma samples from 35 countries, representing all major HIV subtypes, recombinants, and circulating recombinant forms (CRFs) currently in circulation worldwide. Linearity of the Aptima assay was tested on each of 6 major HIV-1 subtypes (A, B, C, D, CRF01_AE, and CRF02_AG) and demonstrated an R(2) value of ≥0.996. The performance of the Aptima assay was also compared to those of the Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 v.2 (CAP/CTM) and Abbott m2000 RealTime HIV-1 (RealTime) assays on all subtype samples. The Aptima assay values averaged 0.21 log higher than the CAP/CTM values and 0.30 log higher than the RealTime values, and the values were >0.4 log higher than CAP/CTM values for subtypes F and G and than RealTime values for subtypes C, F, and G and CRF02_AG. Two samples demonstrated results with >1-log differences from RealTime results. When the data were adjusted by the average difference, 94.9% and 87.0% of Aptima results fell within 0.5 log of the CAP/CTM and RealTime results, respectively. The linearity and accuracy of the Aptima assay in correctly quantitating all major HIV-1 subtypes, coupled with the completely automated format and high throughput of the Panther system, make this system well suited for reliable measurement of viral load in the clinical laboratory.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Analytical characteristics and comparative evaluation of Aptima HCV quant Dx assay with the Abbott RealTime HCV assay and Roche COBAS AmpliPrep/COBAS TaqMan HCV quantitative test v2.0.Virol J. 2017 Apr 4;14(1):66. doi: 10.1186/s12985-017-0727-3. Virol J. 2017. PMID: 28372576 Free PMC article.
-
Multicenter Evaluation of Two Next-Generation HIV-1 Quantitation Assays, Aptima Quant Dx and Cobas 6800, in Comparison to the RealTime HIV-1 Reference Assay.J Clin Microbiol. 2018 Sep 25;56(10):e00292-18. doi: 10.1128/JCM.00292-18. Print 2018 Oct. J Clin Microbiol. 2018. PMID: 30068537 Free PMC article.
-
Analytical characteristics and comparative evaluation of Aptima HIV-1 Quant Dx assay with Ampliprep/COBAS TaqMan HIV-1 test v2.0.Virol J. 2016 Oct 21;13(1):176. doi: 10.1186/s12985-016-0627-y. Virol J. 2016. PMID: 27769309 Free PMC article.
-
Comparative performance of the new Aptima HIV-1 Quant Dx assay with three commercial PCR-based HIV-1 RNA quantitation assays.J Clin Virol. 2015 Aug;69:56-62. doi: 10.1016/j.jcv.2015.05.020. Epub 2015 May 29. J Clin Virol. 2015. PMID: 26209380
-
Evaluating the aptima HIV-1 quant Dx, HCV quant Dx and HBV quant assays against the Abbott HIV-1, HCV and HBV RealTime assays.J Clin Virol. 2018 Sep;106:7-10. doi: 10.1016/j.jcv.2018.06.015. Epub 2018 Jun 25. J Clin Virol. 2018. PMID: 30007139
Cited by
-
The Development of a Standardized Quality Assessment Material to Support Xpert® HIV-1 Viral Load Testing for ART Monitoring in South Africa.Diagnostics (Basel). 2021 Jan 22;11(2):160. doi: 10.3390/diagnostics11020160. Diagnostics (Basel). 2021. PMID: 33499162 Free PMC article.
-
Comparison of the performance of Aptima HIV-1 Quant Dx Assay with Abbott RealTime HIV Assay for viral load monitoring using plasma and Dried Blood Spots collected in Kenya.PLoS One. 2022 Aug 22;17(8):e0269838. doi: 10.1371/journal.pone.0269838. eCollection 2022. PLoS One. 2022. PMID: 35994447 Free PMC article.
-
Forty Years of Molecular Diagnostics for Infectious Diseases.J Clin Microbiol. 2022 Oct 19;60(10):e0244621. doi: 10.1128/jcm.02446-21. Epub 2022 Jul 19. J Clin Microbiol. 2022. PMID: 35852340 Free PMC article. Review.
-
Comparison of the Aptima HIV-1 Quant Dx assay with the COBAS AmpliPrep/COBAS TaqMan HIV-1 v2.0 Test for HIV-1 viral load quantification in plasma samples from HIV-1-infected patients.Health Sci Rep. 2018 Mar 13;1(4):e31. doi: 10.1002/hsr2.31. eCollection 2018 Apr. Health Sci Rep. 2018. PMID: 30623066 Free PMC article.
-
Development of a sensitive, quantitative assay with broad subtype specificity for detection of total HIV-1 nucleic acids in plasma and PBMC.Sci Rep. 2022 Jan 28;12(1):1550. doi: 10.1038/s41598-021-03016-1. Sci Rep. 2022. PMID: 35091568 Free PMC article.
References
-
- World Health Organization (WHO). 2014. March 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/pub/guidelines/arv2013/arvs2013upplement_march201... Accessed 20 February 2016.
-
- Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents. 28 January 2016. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 16 February 2016.
-
- Williams I, Churchill D, Anderson J, Boffito M, Bower M, Cairns G, Cwynarski K, Edwards S, Fidler S, Fisher M, Freedman A, Geretti AM, Gilleece Y, Horne R, Johnson M, Khoo S, Leen C, Marshall N, Nelson M, Orkin C, Paton N, Phillips A, Post F, Pozniak A, Sabin C, Trevelion R, Ustianowski A, Walsh J, Waters L, Wilkins E, Winston A, Youle M. 2014. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012. (Updated November 2013. All changed text is cast in yellow highlight.) HIV Med 15(Suppl 1):S1–S85. doi:10.1111/hiv.12119. - DOI - PubMed
-
- European AIDS Clinical Society. October 2015. EACS guidelines, version 8.0. http://www.eacsociety.org/guidelines/eacs-guidelines/eacsguidelines.html. Accessed 26 February 2016.
-
- Eron JJ, Cooper DA, Steigbigel RT, Clotet B, Gatell JM, Kumar PN, Rockstroh JK, Schechter M, Markowitz M, Yeni P, Loutfy MR, Lazzarin A, Lennox JL, Strohmaier KM, Wan H, Barnard RJ, Nguyen BY, Teppler H, BENCHMRK Study Teams . 2013. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis 13:587–596. doi:10.1016/S1473-3099(13)70093-8. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

