Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy
- PMID: 27510901
- PMCID: PMC6668627
- DOI: 10.1126/scitranslmed.aae0501
Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy
Abstract
Hematopoietic stem cell (HSC) transplantation can cure diverse diseases of the blood system, including hematologic malignancies, anemias, and autoimmune disorders. However, patients must undergo toxic conditioning regimens that use chemotherapy and/or radiation to eliminate host HSCs and enable donor HSC engraftment. Previous studies have shown that anti-c-Kit monoclonal antibodies deplete HSCs from bone marrow niches, allowing donor HSC engraftment in immunodeficient mice. We show that host HSC clearance is dependent on Fc-mediated antibody effector functions, and enhancing effector activity through blockade of CD47, a myeloid-specific immune checkpoint, extends anti-c-Kit conditioning to fully immunocompetent mice. The combined treatment leads to elimination of >99% of host HSCs and robust multilineage blood reconstitution after HSC transplantation. This targeted conditioning regimen that uses only biologic agents has the potential to transform the practice of HSC transplantation and enable its use in a wider spectrum of patients.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
Figures

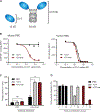
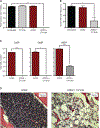
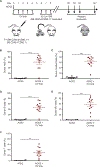
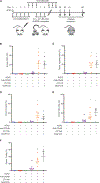
Comment in
-
Devouring the Hematopoietic Stem Cell: Setting the Table for Marrow Cell Transplantation.Mol Ther. 2016 Nov;24(11):1892-1894. doi: 10.1038/mt.2016.193. Mol Ther. 2016. PMID: 27916993 Free PMC article. No abstract available.
References
-
- Shizuru JA, Negrin RS, Weissman IL, Hematopoietic stem and progenitor cells: Clinical and preclinical regeneration of the hematolymphoid system. Annu. Rev. Med 56, 509–538 (2005). - PubMed
-
- Chan CKF, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, Lu W-J, Senarath-Yapa K, Chung MT, Marecic O, Tran M, Yan KS, Upton R, Walmsley GG, Lee AS, Sahoo D, Kuo CJ, Weissman IL, Longaker MT, Identification and specification of the mouse skeletal stem cell. Cell 160, 285–298 (2015). - PMC - PubMed
-
- Deeg HJ; Seattle Marrow Transplant Team, Acute and delayed toxicities of total body irradiation. Int. J. Radiat. Oncol. Biol. Phys 9, 1933–1939 (1983). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

