Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema
- PMID: 27510903
- PMCID: PMC5351811
- DOI: 10.1126/scitranslmed.aaf7837
Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema
Abstract
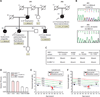
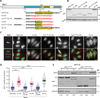
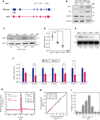
Chronic obstructive pulmonary disease and pulmonary fibrosis have been hypothesized to represent premature aging phenotypes. At times, they cluster in families, but the genetic basis is not understood. We identified rare, frameshift mutations in the gene for nuclear assembly factor 1, NAF1, a box H/ACA RNA biogenesis factor, in pulmonary fibrosis-emphysema patients. The mutations segregated with short telomere length, low telomerase RNA levels, and extrapulmonary manifestations including myelodysplastic syndrome and liver disease. A truncated NAF1 was detected in cells derived from patients, and, in cells in which the frameshift mutation was introduced by genome editing, telomerase RNA levels were reduced. The mutant NAF1 lacked a conserved carboxyl-terminal motif, which we show is required for nuclear localization. To understand the disease mechanism, we used CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 (CRISPR-associated protein-9 nuclease) to generate Naf1(+/-) mice and found that they had half the levels of telomerase RNA. Other box H/ACA RNA levels were also decreased, but rRNA pseudouridylation, which is guided by snoRNAs, was intact. Moreover, first-generation Naf1(+/-) mice showed no evidence of ribosomal pathology. Our data indicate that disease in NAF1 mutation carriers is telomere-mediated; they show that NAF1 haploinsufficiency selectively disturbs telomere length homeostasis by decreasing the levels of telomerase RNA while sparing rRNA pseudouridylation.
Copyright © 2016, American Association for the Advancement of Science.
Figures

References
-
- Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: Final data for 2013. Natl. Vital Stat. Rep. 2016;64:1–119. - PubMed
-
- Silverman EK, Weiss ST, Drazen JM, Chapman HA, Carey V, Campbell EJ, Denish P, Silverman RA, Celedon JC, Reilly JJ, Ginns LC, Speizer FE. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2000;162:2152–2158. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

