Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles
- PMID: 27510922
- PMCID: PMC4987534
- DOI: 10.1038/ncomms12420
Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles
Abstract
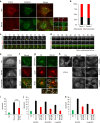
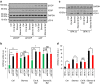
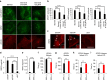
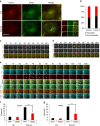
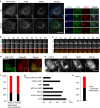
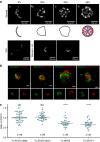
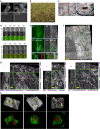
Autophagosome formation requires sequential translocation of autophagy-specific proteins to membranes enriched in PI3P and connected to the ER. Preceding this, the earliest autophagy-specific structure forming de novo is a small punctum of the ULK1 complex. The provenance of this structure and its mode of formation are unknown. We show that the ULK1 structure emerges from regions, where ATG9 vesicles align with the ER and its formation requires ER exit and coatomer function. Super-resolution microscopy reveals that the ULK1 compartment consists of regularly assembled punctate elements that cluster in progressively larger spherical structures and associates uniquely with the early autophagy machinery. Correlative electron microscopy after live imaging shows tubulovesicular membranes present at the locus of this structure. We propose that the nucleation of autophagosomes occurs in regions, where the ULK1 complex coalesces with ER and the ATG9 compartment.
Figures



References
-
- Burman C. & Ktistakis N. T. Autophagosome formation in mammalian cells. Semin. Immunopathol. 32, 397–413 (2010). - PubMed
-
- Lionaki E., Markaki M. & Tavernarakis N. Autophagy and ageing: insights from invertebrate model organisms. Ageing Res. Rev. 12, 413–428 (2013). - PubMed
-
- Mizushima N. & Komatsu M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011). - PubMed
-
- Harris H. & Rubinsztein D. C. Control of autophagy as a therapy for neurodegenerative disease. Nat. Rev. Neurol. 8, 108–117 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

