A novel small molecule that disrupts a key event during the oocyte-to-embryo transition in C. elegans
- PMID: 27510972
- PMCID: PMC5087616
- DOI: 10.1242/dev.140046
A novel small molecule that disrupts a key event during the oocyte-to-embryo transition in C. elegans
Abstract
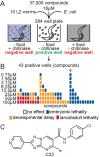
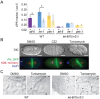
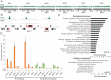
The complex cellular events that occur in response to fertilization are essential for mediating the oocyte-to-embryo transition. Here, we describe a comprehensive small-molecule screen focused on identifying compounds that affect early embryonic events in Caenorhabditis elegans We identify a single novel compound that disrupts early embryogenesis with remarkable stage and species specificity. The compound, named C22, primarily impairs eggshell integrity, leading to osmotic sensitivity and embryonic lethality. The C22-induced phenotype is dependent upon the upregulation of the LET-607/CREBH transcription factor and its candidate target genes, which primarily encode factors involved in diverse aspects of protein trafficking. Together, our data suggest that in the presence of C22, one or more key components of the eggshell are inappropriately processed, leading to permeable, inviable embryos. The remarkable specificity and reversibility of this compound will facilitate further investigation into the role and regulation of protein trafficking in the early embryo, as well as serve as a tool for manipulating the life cycle for other studies such as those involving aging.
Keywords: C. elegans; Oocyte-to-embryo transition; Secretory pathway; Small molecule.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



Similar articles
-
VHA-19 is essential in Caenorhabditis elegans oocytes for embryogenesis and is involved in trafficking in oocytes.PLoS One. 2012;7(7):e40317. doi: 10.1371/journal.pone.0040317. Epub 2012 Jul 2. PLoS One. 2012. PMID: 22768351 Free PMC article.
-
A gain-of-function mutation in oma-1, a C. elegans gene required for oocyte maturation, results in delayed degradation of maternal proteins and embryonic lethality.Dev Biol. 2003 Jun 1;258(1):226-39. doi: 10.1016/s0012-1606(03)00119-2. Dev Biol. 2003. PMID: 12781695
-
ERK activation dynamics in maturing oocyte controls embryonic nuclear divisions in Caenorhabditis elegans.Cell Rep. 2025 Jan 28;44(1):115157. doi: 10.1016/j.celrep.2024.115157. Epub 2025 Jan 9. Cell Rep. 2025. PMID: 39792558 Free PMC article.
-
Control of the oocyte-to-embryo transition by the ubiquitin-proteolytic system in mouse and C. elegans.Curr Opin Cell Biol. 2010 Dec;22(6):758-63. doi: 10.1016/j.ceb.2010.09.003. Epub 2010 Oct 11. Curr Opin Cell Biol. 2010. PMID: 20943362 Review.
-
The oocyte-to-embryo transition.Adv Exp Med Biol. 2013;757:351-72. doi: 10.1007/978-1-4614-4015-4_12. Adv Exp Med Biol. 2013. PMID: 22872483 Review.
Cited by
-
Quantitative Approaches for Scoring in vivo Neuronal Aggregate and Organelle Extrusion in Large Exopher Vesicles in C. elegans.J Vis Exp. 2020 Sep 18;(163):10.3791/61368. doi: 10.3791/61368. J Vis Exp. 2020. PMID: 33016946 Free PMC article.
-
Microfluidic approach to correlate C. elegans neuronal functional aging and underlying changes of gene expression in mechanosensation.Lab Chip. 2024 May 14;24(10):2811-2824. doi: 10.1039/d3lc01080e. Lab Chip. 2024. PMID: 38700452 Free PMC article.
-
Phosphatidylcholine mediates the crosstalk between LET-607 and DAF-16 stress response pathways.PLoS Genet. 2021 May 20;17(5):e1009573. doi: 10.1371/journal.pgen.1009573. eCollection 2021 May. PLoS Genet. 2021. PMID: 34014977 Free PMC article.
-
A simple culture system for long-term imaging of individual C. elegans.Lab Chip. 2017 Nov 7;17(22):3909-3920. doi: 10.1039/c7lc00916j. Lab Chip. 2017. PMID: 29063084 Free PMC article.
-
Development and optimization of a high-throughput screening method utilizing Ancylostoma ceylanicum egg hatching to identify novel anthelmintics.PLoS One. 2019 Jun 3;14(6):e0217019. doi: 10.1371/journal.pone.0217019. eCollection 2019. PLoS One. 2019. PMID: 31158236 Free PMC article.
References
-
- Anderson E. N., Corkins M. E., Li J.-C., Singh K., Parsons S., Tucey T. M., Sorkaç A., Huang H., Dimitriadi M., Sinclair D. A. et al. (2016). C. elegans lifespan extension by osmotic stress requires FUdR, base excision repair, FOXO, and sirtuins. Mech. Ageing Dev. 154, 30-42. 10.1016/j.mad.2016.01.004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

