Leucine Zipper-bearing Kinase promotes axon growth in mammalian central nervous system neurons
- PMID: 27511108
- PMCID: PMC4980599
- DOI: 10.1038/srep31482
Leucine Zipper-bearing Kinase promotes axon growth in mammalian central nervous system neurons
Abstract
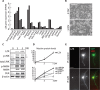
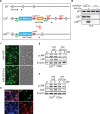
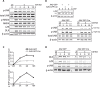
Leucine Zipper-bearing Kinase (LZK/MAP3K13) is a member of the mixed lineage kinase family with high sequence identity to Dual Leucine Zipper Kinase (DLK/MAP3K12). While DLK is established as a key regulator of axonal responses to injury, the role of LZK in mammalian neurons is poorly understood. By gain- and loss-of-function analyses in neuronal cultures, we identify LZK as a novel positive regulator of axon growth. LZK signals specifically through MKK4 and JNKs among MAP2Ks and MAPKs respectively in neuronal cells, with JNK activity positively regulating LZK protein levels. Neuronal maturation or activity deprivation activates the LZK-MKK4-JNK pathway. LZK and DLK share commonalities in signaling, regulation, and effects on axon extension. Furthermore, LZK-dependent regulation of DLK protein expression and the lack of additive effects on axon growth upon co-manipulation suggest complex functional interaction and cross-regulation between these two kinases. Together, our data support the possibility for two structurally related MAP3Ks to work in concert to mediate axonal responses to external insult or injury in mammalian CNS neurons.
Figures




References
-
- Sakuma H. et al.. Molecular cloning and functional expression of a cDNA encoding a new member of mixed lineage protein kinase from human brain. J. Biol. Chem. 272, 28622–28629 (1997). - PubMed
-
- Gallo K. A. & Johnson G. L. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 3, 663–672 (2002). - PubMed
-
- Nihalani D., Merritt S. & Holzman L. B. Identification of structural and functional domains in mixed lineage kinase dual leucine zipper-bearing kinase required for complex formation and stress-activated protein kinase activation. J. Biol. Chem. 275, 7273–7279 (2000). - PubMed
-
- Nakata K. et al.. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 120, 407–420 (2005). - PubMed
-
- Collins C. A., Wairkar Y. P., Johnson S. L. & DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron 51, 57–69 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

