Diffusible Factors Secreted by Glioblastoma and Medulloblastoma Cells Induce Oxidative Stress in Bystander Neural Stem Progenitors
- PMID: 27511909
- PMCID: PMC4984487
- DOI: 10.1177/1759091416662808
Diffusible Factors Secreted by Glioblastoma and Medulloblastoma Cells Induce Oxidative Stress in Bystander Neural Stem Progenitors
Abstract
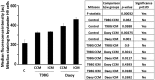
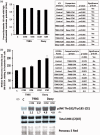
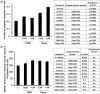
Harmful effects that alter the homeostasis of neural stem or progenitor cells (NSPs) can affect regenerative processes in the central nervous system. We investigated the effect of soluble factors secreted by control or (137)Cs-γ-irradiated glioblastoma or medulloblastoma cells on redox-modulated endpoints in recipient human NSPs. Growth medium harvested from the nonirradiated brain tumor cells, following 24 h of growth, induced prominent oxidative stress in recipient NSPs as judged by overall increases in mitochondrial superoxide radical levels (p < .001), activation of c-jun N-terminal kinase, and decrease in the active form of FoxO3a. The induced oxidative stress was associated with phosphorylation of p53 on serine 15, a marker of DNA damage, induction of the cyclin-cyclin dependent kinase inhibitors p21(Waf1) and p27(Kip1), and perturbations in cell cycle progression (p < .001). These changes were also associated with increased apoptosis as determined by enhanced annexin V staining (p < .001) and caspase 8 activation (p < .05) and altered expression of critical regulators of self-renewal, proliferation, and differentiation. Exposure of the tumor cells to radiation only slightly altered the induced oxidative changes in the bystander NSPs, except for medium from irradiated medulloblastoma cells that was more potent at inducing apoptosis in the NSPs than medium from nonirradiated cells (p < .001). The elucidation of such stressful bystander effects provides avenues to understand the biochemical events underlying the development or exacerbation of degenerative outcomes associated with brain cancers. It is also relevant to tissue culture protocols whereby growth medium conditioned by tumor cells is often used to support the growth of stem cells.
Keywords: brain tumors; intercellular communication; ionizing radiation or bystander effect; neural stem progenitors; reactive oxygen species.
© The Author(s) 2016.
Figures




References
-
- Acharya M. M., Lan M. L., Kan V. H., Patel N. H., Giedzinski E., Tseng B. P., Limoli C. L. (2010) Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radical Biology & Medicine 49: 1846–1855. - PubMed
-
- Azzam E. I., De Toledo S. M., Harris A. L., Ivanov V., Zhou H., Amundson S. A., Hei T. K. (2013) The ionizing radiation-induced bystander effect: Evidence, mechanism and significance. In: Sonis S. T., Keefe D. M. (eds) Pathobiology of cancer regimen-related toxicities, New York, NY: Springer, pp. 42–68.
-
- Azzam E. I., de Toledo S. M., Little J. B. (2003) Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene 22: 7050–7057. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
