ARID3B: a Novel Regulator of the Kaposi's Sarcoma-Associated Herpesvirus Lytic Cycle
- PMID: 27512077
- PMCID: PMC5044832
- DOI: 10.1128/JVI.03262-15
ARID3B: a Novel Regulator of the Kaposi's Sarcoma-Associated Herpesvirus Lytic Cycle
Abstract
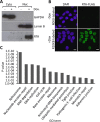
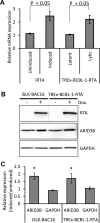
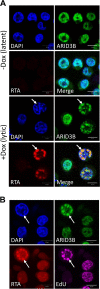
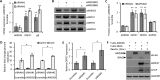
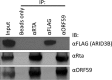
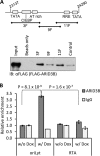
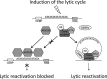
Kaposi's sarcoma-associated herpesvirus (KSHV) is the causative agent of commonly fatal malignancies of immunocompromised individuals, including primary effusion lymphoma (PEL) and Kaposi's sarcoma (KS). A hallmark of all herpesviruses is their biphasic life cycle-viral latency and the productive lytic cycle-and it is well established that reactivation of the KSHV lytic cycle is associated with KS pathogenesis. Therefore, a thorough appreciation of the mechanisms that govern reactivation is required to better understand disease progression. The viral protein replication and transcription activator (RTA) is the KSHV lytic switch protein due to its ability to drive the expression of various lytic genes, leading to reactivation of the entire lytic cycle. While the mechanisms for activating lytic gene expression have received much attention, how RTA impacts cellular function is less well understood. To address this, we developed a cell line with doxycycline-inducible RTA expression and applied stable isotope labeling of amino acids in cell culture (SILAC)-based quantitative proteomics. Using this methodology, we have identified a novel cellular protein (AT-rich interacting domain containing 3B [ARID3B]) whose expression was enhanced by RTA and that relocalized to replication compartments upon lytic reactivation. We also show that small interfering RNA (siRNA) knockdown or overexpression of ARID3B led to an enhancement or inhibition of lytic reactivation, respectively. Furthermore, DNA affinity and chromatin immunoprecipitation assays demonstrated that ARID3B specifically interacts with A/T-rich elements in the KSHV origin of lytic replication (oriLyt), and this was dependent on lytic cycle reactivation. Therefore, we have identified a novel cellular protein whose expression is enhanced by KSHV RTA with the ability to inhibit KSHV reactivation.
Importance: Kaposi's sarcoma-associated herpesvirus (KSHV) is the causative agent of fatal malignancies of immunocompromised individuals, including Kaposi's sarcoma (KS). Herpesviruses are able to establish a latent infection, in which they escape immune detection by restricting viral gene expression. Importantly, however, reactivation of productive viral replication (the lytic cycle) is necessary for the pathogenesis of KS. Therefore, it is important that we comprehensively understand the mechanisms that govern lytic reactivation, to better understand disease progression. In this study, we have identified a novel cellular protein (AT-rich interacting domain protein 3B [ARID3B]) that we show is able to temper lytic reactivation. We showed that the master lytic switch protein, RTA, enhanced ARID3B levels, which then interacted with viral DNA in a lytic cycle-dependent manner. Therefore, we have added a new factor to the list of cellular proteins that regulate the KSHV lytic cycle, which has implications for our understanding of KSHV biology.
Copyright © 2016 Wood et al.
Figures
Similar articles
-
Sirtuin 6 Attenuates Kaposi's Sarcoma-Associated Herpesvirus Reactivation by Suppressing Ori-Lyt Activity and Expression of RTA.J Virol. 2019 Mar 21;93(7):e02200-18. doi: 10.1128/JVI.02200-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651359 Free PMC article.
-
Genome-Wide Identification of Direct RTA Targets Reveals Key Host Factors for Kaposi's Sarcoma-Associated Herpesvirus Lytic Reactivation.J Virol. 2019 Feb 19;93(5):e01978-18. doi: 10.1128/JVI.01978-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541837 Free PMC article.
-
Activation of Kaposi's sarcoma-associated herpesvirus (KSHV) by inhibitors of class III histone deacetylases: identification of sirtuin 1 as a regulator of the KSHV life cycle.J Virol. 2014 Jun;88(11):6355-67. doi: 10.1128/JVI.00219-14. Epub 2014 Mar 26. J Virol. 2014. PMID: 24672028 Free PMC article.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
-
Epigenetic regulation of Kaposi's sarcoma-associated herpesvirus replication.Semin Cancer Biol. 2009 Jun;19(3):153-7. doi: 10.1016/j.semcancer.2009.02.010. Epub 2009 Feb 21. Semin Cancer Biol. 2009. PMID: 19429478 Free PMC article. Review.
Cited by
-
Merkel Cell Polyomavirus Small T Antigen Drives Cell Motility via Rho-GTPase-Induced Filopodium Formation.J Virol. 2018 Jan 2;92(2):e00940-17. doi: 10.1128/JVI.00940-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093086 Free PMC article.
-
Proteomic Applications in Antimicrobial Resistance and Clinical Microbiology Studies.Infect Drug Resist. 2020 Jun 16;13:1785-1806. doi: 10.2147/IDR.S238446. eCollection 2020. Infect Drug Resist. 2020. PMID: 32606829 Free PMC article. Review.
-
Bayes Factor-Based Regulatory Gene Network Analysis of Genome-Wide Association Study of Economic Traits in a Purebred Swine Population.Genes (Basel). 2019 Apr 10;10(4):293. doi: 10.3390/genes10040293. Genes (Basel). 2019. PMID: 30974885 Free PMC article.
-
Impact of HVT Vaccination on Splenic miRNA Expression in Marek's Disease Virus Infections.Genes (Basel). 2019 Feb 5;10(2):115. doi: 10.3390/genes10020115. Genes (Basel). 2019. PMID: 30764490 Free PMC article.
-
LIN28-let-7 axis regulates genes in immortalized human trophoblast cells by targeting the ARID3B-complex.FASEB J. 2019 Nov;33(11):12348-12363. doi: 10.1096/fj.201900718RR. Epub 2019 Aug 15. FASEB J. 2019. PMID: 31415216 Free PMC article.
References
-
- Quinlivan EB, Zhang C, Stewart PW, Komoltri C, Davis MG, Wehbie RS. 2002. Elevated virus loads of Kaposi's sarcoma-associated human herpesvirus 8 predict Kaposi's sarcoma disease progression, but elevated levels of human immunodeficiency virus type 1 do not. J Infect Dis 185:1736–1744. doi:10.1086/340652. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

