IL-1β/HMGB1 signalling promotes the inflammatory cytokines release via TLR signalling in human intervertebral disc cells
- PMID: 27512095
- PMCID: PMC5025813
- DOI: 10.1042/BSR20160118
IL-1β/HMGB1 signalling promotes the inflammatory cytokines release via TLR signalling in human intervertebral disc cells
Abstract
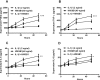
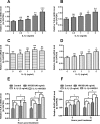
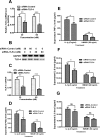
Inflammation and cytokines have been recognized to correlate with intervertebral disc (IVD) degeneration (IDD), via mediating the development of clinical signs and symptoms. However, the regulation mechanism remains unclear. We aimed at investigating the regulatory role of interleukin (IL)β and high mobility group box 1 (HMGB1) in the inflammatory response in human IVD cells, and then explored the signalling pathways mediating such regulatory effect. Firstly, the promotion to inflammatory cytokines in IVD cells was examined with ELISA method. And then western blot and real time quantitative PCR were performed to analyse the expression of toll-like receptors (TLRs), receptors for advanced glycation endproducts (RAGE) and NF-κB signalling markers in the IL-1β- or (and) HMGB1-treated IVD cells. Results demonstrated that either IL-1β or HMGB1 promoted the release of the inflammatory cytokines such as prostaglandin E2 (PGE2), TNF-α, IL-6 and IL-8 in human IVD cells. And the expression of matrix metalloproteinases (MMPs) such as MMP-1, -3 and -9 was also additively up-regulated by IL-1β and HMGB1. We also found such additive promotion to the expression of TLR-2, TLR-4 and RAGE, and the NF-κB signalling in intervertebral disc cells. In summary, our study demonstrated that IL-1β and HMGB1 additively promotes the release of inflammatory cytokines and the expression of MMPs in human IVD cells. The TLRs and RAGE and the NF-κB signalling were also additively promoted by IL-1β and HMGB1. Our study implied that the additive promotion by IL-1β and HMGB1 to inflammatory cytokines and MMPs might aggravate the progression of IDD.
Keywords: high mobility group box 1 (HMGB1); interleukin (IL)β; intervertebral disc degeneration (IDD).
© 2016 The Author(s).
Figures



Similar articles
-
Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells.Eur Spine J. 2014 Sep;23(9):1878-91. doi: 10.1007/s00586-014-3442-4. Epub 2014 Jul 5. Eur Spine J. 2014. PMID: 24997157
-
Toll-Like Receptor 4 (TLR4) expression and stimulation in a model of intervertebral disc inflammation and degeneration.Spine (Phila Pa 1976). 2013 Jul 15;38(16):1343-51. doi: 10.1097/BRS.0b013e31826b71f4. Spine (Phila Pa 1976). 2013. PMID: 22850250
-
High mobility group box-1 induces pro-inflammatory signaling in human nucleus pulposus cells via toll-like receptor 4-dependent pathway.J Orthop Res. 2019 Jan;37(1):220-231. doi: 10.1002/jor.24154. Epub 2018 Oct 29. J Orthop Res. 2019. PMID: 30273982 Free PMC article.
-
Expatiating the molecular approaches of HMGB1 in diabetes mellitus: Highlighting signalling pathways via RAGE and TLRs.Mol Biol Rep. 2021 Feb;48(2):1869-1881. doi: 10.1007/s11033-020-06130-x. Epub 2021 Jan 21. Mol Biol Rep. 2021. PMID: 33479829 Review.
-
[Relationship between inflammatory cytokines of IL-1β and TNF-α and intervertebral disc degeneration].Zhongguo Gu Shang. 2017 Sep 25;30(9):866-871. doi: 10.3969/j.issn.1003-0034.2017.09.017. Zhongguo Gu Shang. 2017. PMID: 29455492 Review. Chinese.
Cited by
-
Blocking toll-like receptor 4 mitigates static loading induced pro-inflammatory expression in intervertebral disc motion segments.J Biomech. 2023 Mar;150:111491. doi: 10.1016/j.jbiomech.2023.111491. Epub 2023 Feb 11. J Biomech. 2023. PMID: 36870259 Free PMC article.
-
Signaling Mechanisms of Stem Cell Therapy for Intervertebral Disc Degeneration.Biomedicines. 2023 Sep 6;11(9):2467. doi: 10.3390/biomedicines11092467. Biomedicines. 2023. PMID: 37760908 Free PMC article. Review.
-
Anti‑inflammatory mechanism of berberine on lipopolysaccharide‑induced IEC‑18 models based on comparative transcriptomics.Mol Med Rep. 2020 Dec;22(6):5163-5180. doi: 10.3892/mmr.2020.11602. Epub 2020 Oct 14. Mol Med Rep. 2020. PMID: 33174609 Free PMC article.
-
Targeting advanced glycation end products: potential therapeutic approaches for mitigating diabetic intervertebral disc degeneration?Front Endocrinol (Lausanne). 2025 Jul 7;16:1618984. doi: 10.3389/fendo.2025.1618984. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40692599 Free PMC article. Review.
-
Intradiscal inflammatory stimulation induces spinal pain behavior and intervertebral disc degeneration in vivo.FASEB J. 2024 Jan;38(1):e23364. doi: 10.1096/fj.202300227R. FASEB J. 2024. PMID: 38091247 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

