Uterotonic Neuromedin U Receptor 2 and Its Ligands Are Upregulated by Inflammation in Mice and Humans, and Elicit Preterm Birth
- PMID: 27512149
- PMCID: PMC5394981
- DOI: 10.1095/biolreprod.116.140905
Uterotonic Neuromedin U Receptor 2 and Its Ligands Are Upregulated by Inflammation in Mice and Humans, and Elicit Preterm Birth
Abstract
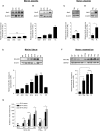
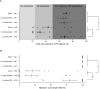
Uterine labor requires the conversion of a quiescent (propregnancy) uterus into an activated (prolabor) uterus, with increased sensitivity to endogenous uterotonic molecules. This activation is induced by stressors, particularly inflammation in term and preterm labor. Neuromedin U (NmU) is a neuropeptide known for its uterocontractile effects in rodents. The objective of the study was to assess the expression and function of neuromedin U receptor 2 (NmU-R2) and its ligands NmU and the more potent neuromedin S (NmS) in gestational tissues, and the possible implication of inflammatory stressors in triggering this system. Our data show that NmU and NmS are uterotonic ex vivo in murine tissue, and they dose-dependently trigger labor by acting specifically via NmU-R2. Expression of NmU-R2, NmU, and NmS is detected in murine and human gestational tissues by immunoblot, and the expression of NmS in placenta and of NmU-R2 in uterus increases considerably with gestation age and labor, which is associated with amplified NmU-induced uterocontractile response in mice. NmU- and NmS-induced contraction is associated with increased NmU-R2-coupled Ca++ transients, and Akt and Erk activation in murine primary myometrial smooth muscle cells (mSMCs), which are potentiated with gestational age. NmU-R2 is upregulated in vitro in mSMCs and in vivo in uterus in response to proinflammatory interleukin 1beta (IL1beta), which is associated with increased NmU-induced uterocontractile response and Ca++ transients in murine and human mSMCs; additionally, placental NmS is markedly upregulated in vivo in response to IL1beta. In human placenta at term, immunohistological analysis revealed NmS expression primarily in cytotrophoblasts; furthermore, stimulation with lipopolysaccharide (LPS; Gram-negative endotoxin) markedly upregulates NmS expression in primary human cytotrophoblasts isolated from term placentas. Correspondingly, decidua of women with clinical signs of infection who delivered preterm display significantly higher expression of NmS compared with those without infection. Importantly, in vivo knockdown of NmU-R2 prevents LPS-triggered preterm birth in mice and the associated neonatal mortality. Altogether, our data suggest a critical role for NmU-R2 and its ligands NmU and NmS in preterm labor triggered by infection. We hereby identify NmU-R2 as a relevant target for preterm birth.
Keywords: NmU-R2; calcium; contraction; infection; inflammation; neuromedin S; neuromedin U; preterm birth; preterm labor; uterine labor.
© 2016 by the Society for the Study of Reproduction, Inc.
Figures




Similar articles
-
Lactate produced during labor modulates uterine inflammation via GPR81 (HCA1).Am J Obstet Gynecol. 2017 Jan;216(1):60.e1-60.e17. doi: 10.1016/j.ajog.2016.09.072. Epub 2016 Sep 9. Am J Obstet Gynecol. 2017. PMID: 27615440
-
Involvement of endogenous neuromedin U and neuromedin S in thermoregulation.Biochem Biophys Res Commun. 2016 Feb 19;470(4):930-5. doi: 10.1016/j.bbrc.2016.01.155. Epub 2016 Jan 27. Biochem Biophys Res Commun. 2016. PMID: 26826380
-
Distinct functions of neuromedin u and neuromedin s in orange-spotted grouper.J Mol Endocrinol. 2015 Oct;55(2):95-106. doi: 10.1530/JME-15-0018. Epub 2015 Jul 10. J Mol Endocrinol. 2015. PMID: 26162607
-
Neuromedins NMU and NMS: An Updated Overview of Their Functions.Front Endocrinol (Lausanne). 2021 Jul 1;12:713961. doi: 10.3389/fendo.2021.713961. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34276571 Free PMC article. Review.
-
Emerging pharmacology and physiology of neuromedin U and the structurally related peptide neuromedin S.Br J Pharmacol. 2009 Sep;158(1):87-103. doi: 10.1111/j.1476-5381.2009.00252.x. Epub 2009 Jun 10. Br J Pharmacol. 2009. PMID: 19519756 Free PMC article. Review.
Cited by
-
The Preventive Effects of Quercetin on Preterm Birth Based on Network Pharmacology and Bioinformatics.Reprod Sci. 2022 Jan;29(1):193-202. doi: 10.1007/s43032-021-00674-4. Epub 2021 Jul 6. Reprod Sci. 2022. PMID: 34231170
-
Spontaneous preterm birth: Involvement of multiple feto-maternal tissues and organ systems, differing mechanisms, and pathways.Front Endocrinol (Lausanne). 2022 Oct 13;13:1015622. doi: 10.3389/fendo.2022.1015622. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36313741 Free PMC article. Review.
-
Cooperative effects of sequential PGF2α and IL-1β on IL-6 and COX-2 expression in human myometrial cells†.Biol Reprod. 2019 May 1;100(5):1370-1385. doi: 10.1093/biolre/ioz029. Biol Reprod. 2019. PMID: 30794283 Free PMC article.
-
Inflammatory Amplification: A Central Tenet of Uterine Transition for Labor.Front Cell Infect Microbiol. 2021 Aug 19;11:660983. doi: 10.3389/fcimb.2021.660983. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34490133 Free PMC article. Review.
-
A comparative analysis of the intrauterine transcriptome in fertile and subfertile mares using cytobrush sampling.BMC Genomics. 2021 May 22;22(1):377. doi: 10.1186/s12864-021-07701-3. BMC Genomics. 2021. PMID: 34022808 Free PMC article.
References
-
- Cook JL, Shallow MC, Zaragoza DB, Anderson KI, Olson DM. Mouse placental prostaglandins are associated with uterine activation and the timing of birth. Biol Reprod. 2003;68:579–587. - PubMed
-
- Bennett P. Preterm labour. In: Edmonds DK, editor. Dewhurst's Textbook of Obstetrics and Gynaecology, 8th ed. Oxford, UK: Wiley-Blackwell; 2012. pp. 338–355. In: (ed.)
-
- Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. - PubMed
-
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition–a review. Placenta. 2003;24(suppl A):S33–S46. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

