Total Radiosynthesis: Thinking outside "the box"
- PMID: 27512156
- PMCID: PMC4976501
- DOI: 10.1071/CH15406
Total Radiosynthesis: Thinking outside "the box"
Abstract
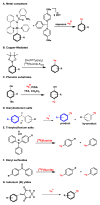
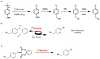
The logic of total synthesis transformed a stagnant state of medicinal and synthetic organic chemistry when there was a paucity of methods and reagents to synthesize drug molecules and/or natural products. Molecular imaging by positron emission tomography (PET) is now experiencing a renaissance in the way radiopharmaceuticals for molecular imaging are synthesized, however, a paradigm shift is desperately needed in the discovery pipeline to accelerate in vivo imaging studies. A significant challenge in radiochemistry is the limited choice of labeled reagents (or building blocks) available for the synthesis of novel radiopharmaceuticals with the most commonly used short-lived radionuclides carbon-11 (11C; half-life ~20 minutes) and fluorine-18 (18F; half-life ~2 hours). In fact, most drugs cannot be labeled with 11C or 18F due to a lack of efficient and diverse radiosynthetic methods. In general, routine radiopharmaceutical production relies on the incorporation of the isotope at the last or penultimate step of synthesis, ideally within one half-life of the radionuclide, to maximize radiochemical yields and specific activities thereby reducing losses due to radioactive decay. Reliance on radiochemistry conducted within the constraints of an automated synthesis unit ("box") has stifled the exploration of multi-step reactions with short-lived radionuclides. Radiopharmaceutical synthesis can be transformed by considering logic of total synthesis to develop novel approaches for 11C- and 18F-radiolabeling complex molecules via retrosynthetic analysis and multi-step reactions. As a result of such exploration, new methods, reagents and radiopharmaceuticals for in vivo imaging studies are discovered. A new avenue to develop radiotracers that were previously unattainable due to the lack of efficient radiosynthetic methods is necessary to work towards our ultimate, albeit impossible goal - the concept we term total radiosynthesis - to radiolabel virtually any molecule. As with the vast majority of drugs, most radiotracers also fail, therefore expeditious evaluation of tracers in preclinical models prior to optimization or derivatization of the lead molecules/drugs is necessary. Furthermore the exact position of the 11C and 18F radionuclide in tracers is often critical for metabolic considerations, and flexible methodologies to introduce the radiolabel are needed. Using the principles of total synthesis our laboratory and others have shown that multi-step radiochemical reactions are indeed suitable for preclinical and even clinical use. As the goal of total synthesis is to be concise, we have also simplified the syntheses of radiopharmaceuticals. We are presently developing new strategies via [11C]CO2 fixation which has enabled library radiosynthesis as well as labeling non-activated arenes using [18F]fluoride via iodonium ylides. Both of which have proven to be suitable for human PET imaging. We concurrently utilize state-of-the-art automation technologies including microfluidic flow chemistry and rapid purification strategies for radiopharmaceutical production. In this account we highlight how total radiosynthesis has impacted our radiochemistry program, with prominent examples from others, focusing on its impact towards preclinical and clinical research studies.
Figures




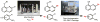
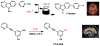
References
-
- Ido T, Wan CN, Casella V, Fowler JS, Wolf AP, Reivich M, Kuhl DE. J Labelled Compd Radiopharm. 1978;14:175–183.
- Fowler JS, Ido T. Semin Nucl Med. 2002;32:6–12. - PubMed
-
- Corey EJ, Cheng X-m. The logic of chemical synthesis. John Wiley; New York: 1989.
- Corey EJ, Czakó B, Kürti L. Molecules and medicine. John Wiley & Sons; Hoboken, N.J: 2007.
-
- Vasdev N, Natesan S, Galineau L, Garcia A, Stableford WT, McCormick P, Seeman P, Houle S, Wilson AA. Synapse. 2006;60:314–318. - PubMed
-
- Wilson AA, Garcia A, Houle S, Sadovski O, Vasdev N. Chem-Eur J. 2011;17:259–264. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
