Approach to Cutaneous Lymphoid Infiltrates: When to Consider Lymphoma?
- PMID: 27512181
- PMCID: PMC4966394
- DOI: 10.4103/0019-5154.185698
Approach to Cutaneous Lymphoid Infiltrates: When to Consider Lymphoma?
Abstract
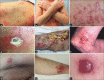
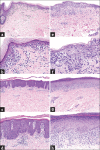
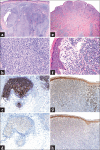
Cutaneous lymphoid infiltrates (CLIs) are common in routine dermatopathology. However, differentiating a reactive CLI from a malignant lymphocytic infiltrate is often a significant challenge since many inflammatory dermatoses can clinically and/or histopathologically mimic cutaneous lymphomas, coined pseudolymphomas. We conducted a literature review from 1966 to July 1, 2015, at PubMed.gov using the search terms: Cutaneous lymphoma, cutaneous pseudolymphoma, cutaneous lymphoid hyperplasia, simulants/mimics/imitators of cutaneous lymphomas, and cutaneous lymphoid infiltrates. The diagnostic approach to CLIs and the most common differential imitators of lymphoma is discussed herein based on six predominant morphologic and immunophenotypic, histopathologic patterns: (1) Superficial dermal T-cell infiltrates (2) superficial and deep dermal perivascular and/or nodular natural killer/T-cell infiltrates (3) pan-dermal diffuse T-cell infiltrates (4) panniculitic T-cell infiltrates (5) small cell predominant B-cell infiltrates, and (6) large-cell predominant B-cell infiltrates. Since no single histopathological feature is sufficient to discern between a benign and a malignant CLI, the overall balance of clinical, histopathological, immunophenotypic, and molecular features should be considered carefully to establish a diagnosis. Despite advances in ancillary studies such as immunohistochemistry and molecular clonality, these studies often display specificity and sensitivity limitations. Therefore, proper clinicopathological correlation still remains the gold standard for the precise diagnosis of CLIs.
Keywords: Cutaneous lymphoid hyperplasia; cutaneous lymphoid infiltrates; cutaneous lymphoma; cutaneous pseudolymphoma; simulants of cutaneous lymphomas.
Figures





Similar articles
-
Approach to dermal-based lymphoid infiltrates and proliferations.Semin Cutan Med Surg. 2018 Mar;37(1):61-74. doi: 10.12788/j.sder.2018.015. Semin Cutan Med Surg. 2018. PMID: 29719022 Review.
-
Atypical lymphoid hyperplasia mimicking lymphoma.Hematol Oncol Clin North Am. 2009 Aug;23(4):729-45. doi: 10.1016/j.hoc.2009.04.005. Hematol Oncol Clin North Am. 2009. PMID: 19577167 Review.
-
Impact of molecular analysis in the diagnosis of cutaneous lymphoid infiltrates.Semin Cutan Med Surg. 2000 Jun;19(2):87-90. doi: 10.1016/s1085-5629(00)80004-8. Semin Cutan Med Surg. 2000. PMID: 10892709 Review.
-
A Systematic Approach to the Cutaneous Lymphoid Infiltrates: A Clinical, Morphologic, and Immunophenotypic Evaluation.Arch Pathol Lab Med. 2019 Aug;143(8):958-979. doi: 10.5858/arpa.2018-0294-RA. Arch Pathol Lab Med. 2019. PMID: 31339758
-
The value of molecular analysis by PCR in the diagnosis of cutaneous lymphocytic infiltrates.J Cutan Pathol. 2002 Sep;29(8):447-52. doi: 10.1034/j.1600-0560.2002.290801.x. J Cutan Pathol. 2002. PMID: 12207737 Review.
Cited by
-
Cutaneous pseudolymphoma: A clinicopathological study and immunohistochemical patterns.Caspian J Intern Med. 2021 Apr;12(3):283-289. doi: 10.22088/cjim.12.3.283. Caspian J Intern Med. 2021. PMID: 34221277 Free PMC article.
-
Cutaneous Lymphomas in India: Prospects and Limitations.Indian J Dermatol. 2017 Mar-Apr;62(2):135-136. doi: 10.4103/ijd.IJD_72_17. Indian J Dermatol. 2017. PMID: 28400631 Free PMC article. No abstract available.
-
Cutaneous Involvement of Extranodal NK/T Cell Lymphoma, Nasal Type, a Clinical and Histopathological Mimicker of Various Skin Diseases.Dermatopathology (Basel). 2022 Sep 9;9(3):307-320. doi: 10.3390/dermatopathology9030037. Dermatopathology (Basel). 2022. PMID: 36135102 Free PMC article.
-
Sustained remission of recalcitrant cutaneous lymphoid hyperplasia after thalidomide treatment.JAAD Case Rep. 2018 Feb 28;4(3):245-247. doi: 10.1016/j.jdcr.2018.01.012. eCollection 2018 Apr. JAAD Case Rep. 2018. PMID: 29687061 Free PMC article. No abstract available.
-
The Puzzle of Papules Over Face and Extrafacial Areas: A Rare Case of Disseminated Idiopathic T-Cell Pseudolymphoma.Indian Dermatol Online J. 2021 Mar 2;12(2):312-315. doi: 10.4103/idoj.IDOJ_568_20. eCollection 2021 Mar-Apr. Indian Dermatol Online J. 2021. PMID: 33959532 Free PMC article.
References
-
- Cerroni L. 4th ed. Hoboken, NJ: Wiley-Blackwell; 2014. Skin Lymphoma: The Illustrated Guide.
-
- Sarantopoulos GP, Palla B, Said J, Kinney MC, Swerdlow SM, Willemze R, et al. Mimics of cutaneous lymphoma: Report of the 2011 Society for Hematopathology/European Association for Haematopathology workshop. Am J Clin Pathol. 2013;139:536–51. - PubMed
-
- Laban É, Beylot-Barry M, Ortonne N, Battistella M, Carlotti A, de Muret A, et al. Cutaneous lymphoproliferations: Proposal for the use of diagnostic algorithms based on 2760 cases of cutaneous lymphoproliferations taken from the INCa networks (LYMPHOPATH and GFELC) over a two-year period. Ann Pathol. 2015;35:131–47. - PubMed
-
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–85. - PubMed
-
- Magro CM, Crowson AN. Drug-induced immune dysregulation as a cause of atypical cutaneous lymphoid infiltrates: A hypothesis. Hum Pathol. 1996;27:125–32. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
