Oncolytic herpes viruses, chemotherapeutics, and other cancer drugs
- PMID: 27512658
- PMCID: PMC4918355
- DOI: 10.2147/OV.S52601
Oncolytic herpes viruses, chemotherapeutics, and other cancer drugs
Abstract
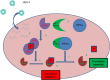
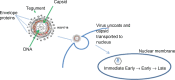
Oncolytic viruses are emerging as a potential new way of treating cancers. They are selectively replication-competent viruses that propagate only in actively dividing tumor cells but not in normal cells and, as a result, destroy the tumor cells by consequence of lytic infection. At least six different oncolytic herpes simplex viruses (oHSVs) have undergone clinical trials worldwide to date, and they have demonstrated an excellent safety profile and intimations of efficacy. The first pivotal Phase III trial with an oHSV, talimogene laherparepvec (T-Vec [OncoVex(GM-CSF)]), is almost complete, with extremely positive early results reported. Intuitively, therapeutically beneficial interactions between oHSV and chemotherapeutic and targeted therapeutic drugs would be limited as the virus requires actively dividing cells for maximum replication efficiency and most anticancer agents are cytotoxic or cytostatic. However, combinations of such agents display a range of responses, with antagonistic, additive, or, perhaps most surprisingly, synergistic enhancement of antitumor activity. When synergistic interactions in cancer cell killing are observed, chemotherapy dose reductions that achieve the same overall efficacy may be possible, resulting in a valuable reduction of adverse side effects. Therefore, the combination of an oHSV with "standard-of-care" drugs makes a logical and reasonable approach to improved therapy, and the addition of a targeted oncolytic therapy with "standard-of-care" drugs merits further investigation, both preclinically and in the clinic. Numerous publications report such studies of oncolytic HSV in combination with other drugs, and we review their findings here. Viral interactions with cellular hosts are complex and frequently involve intracellular signaling networks, thus creating diverse opportunities for synergistic or additive combinations with many anticancer drugs. We discuss potential mechanisms that may lead to synergistic interactions.
Keywords: combination studies; herpes simplex virus; oncolytic virus; virotherapy.
Figures

Similar articles
-
Oncolytic Virotherapy by HSV.Adv Exp Med Biol. 2018;1045:63-84. doi: 10.1007/978-981-10-7230-7_4. Adv Exp Med Biol. 2018. PMID: 29896663 Review.
-
Oncolytic herpes simplex virus and immunotherapy.BMC Immunol. 2018 Dec 18;19(1):40. doi: 10.1186/s12865-018-0281-9. BMC Immunol. 2018. PMID: 30563466 Free PMC article. Review.
-
The Oncolytic Herpes Simplex Virus Talimogene Laherparepvec Shows Promising Efficacy in Neuroendocrine Cancer Cell Lines.Neuroendocrinology. 2019;109(4):346-361. doi: 10.1159/000500159. Epub 2019 Jun 13. Neuroendocrinology. 2019. PMID: 31280274
-
Construction of Oncolytic Herpes Simplex Virus with Therapeutic Genes of Interest.Methods Mol Biol. 2019;1937:177-188. doi: 10.1007/978-1-4939-9065-8_10. Methods Mol Biol. 2019. PMID: 30706396
-
Oncolytic herpes simplex virus vectors and chemotherapy: are combinatorial strategies more effective for cancer?Future Oncol. 2010 Apr;6(4):619-34. doi: 10.2217/fon.10.18. Future Oncol. 2010. PMID: 20373873 Free PMC article. Review.
Cited by
-
Progression of oncolytic virus in liver cancer treatment.Front Oncol. 2024 Sep 26;14:1446085. doi: 10.3389/fonc.2024.1446085. eCollection 2024. Front Oncol. 2024. PMID: 39391253 Free PMC article. Review.
-
Chemotherapy and Oncolytic Virotherapy: Advanced Tactics in the War against Cancer.Front Oncol. 2014 Jun 11;4:145. doi: 10.3389/fonc.2014.00145. eCollection 2014. Front Oncol. 2014. PMID: 24967214 Free PMC article. Review.
-
Blockade of transforming growth factor-β signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models.Int J Cancer. 2017 Dec 1;141(11):2348-2358. doi: 10.1002/ijc.30929. Epub 2017 Aug 26. Int J Cancer. 2017. PMID: 28801914 Free PMC article.
References
-
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–659. - PubMed
-
- Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–856. - PubMed
-
- Campadelli-Fiume G, De Giovanni C, Gatta V, Nanni P, Lollini PL, Menotti L. Rethinking herpes simplex virus: the way to oncolytic agents. Rev Med Virol. 2011;21(4):213–226. - PubMed
-
- Mineta T, Rabkin SD, Martuza RL. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994;54(15):3963–3966. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

