Combating a Global Threat to a Clonal Crop: Banana Black Sigatoka Pathogen Pseudocercospora fijiensis (Synonym Mycosphaerella fijiensis) Genomes Reveal Clues for Disease Control
- PMID: 27512984
- PMCID: PMC4981457
- DOI: 10.1371/journal.pgen.1005876
Combating a Global Threat to a Clonal Crop: Banana Black Sigatoka Pathogen Pseudocercospora fijiensis (Synonym Mycosphaerella fijiensis) Genomes Reveal Clues for Disease Control
Erratum in
-
Correction: Combating a Global Threat to a Clonal Crop: Banana Black Sigatoka Pathogen Pseudocercospora fijiensis (Synonym Mycosphaerella fijiensis) Genomes Reveal Clues for Disease Control.PLoS Genet. 2016 Oct 4;12(10):e1006365. doi: 10.1371/journal.pgen.1006365. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27701412 Free PMC article.
Abstract
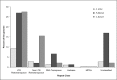
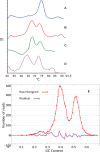
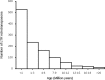
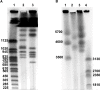
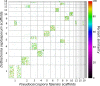
Black Sigatoka or black leaf streak disease, caused by the Dothideomycete fungus Pseudocercospora fijiensis (previously: Mycosphaerella fijiensis), is the most significant foliar disease of banana worldwide. Due to the lack of effective host resistance, management of this disease requires frequent fungicide applications, which greatly increase the economic and environmental costs to produce banana. Weekly applications in most banana plantations lead to rapid evolution of fungicide-resistant strains within populations causing disease-control failures throughout the world. Given its extremely high economic importance, two strains of P. fijiensis were sequenced and assembled with the aid of a new genetic linkage map. The 74-Mb genome of P. fijiensis is massively expanded by LTR retrotransposons, making it the largest genome within the Dothideomycetes. Melting-curve assays suggest that the genomes of two closely related members of the Sigatoka disease complex, P. eumusae and P. musae, also are expanded. Electrophoretic karyotyping and analyses of molecular markers in P. fijiensis field populations showed chromosome-length polymorphisms and high genetic diversity. Genetic differentiation was also detected using neutral markers, suggesting strong selection with limited gene flow at the studied geographic scale. Frequencies of fungicide resistance in fungicide-treated plantations were much higher than those in untreated wild-type P. fijiensis populations. A homologue of the Cladosporium fulvum Avr4 effector, PfAvr4, was identified in the P. fijiensis genome. Infiltration of the purified PfAVR4 protein into leaves of the resistant banana variety Calcutta 4 resulted in a hypersensitive-like response. This result suggests that Calcutta 4 could carry an unknown resistance gene recognizing PfAVR4. Besides adding to our understanding of the overall Dothideomycete genome structures, the P. fijiensis genome will aid in developing fungicide treatment schedules to combat this pathogen and in improving the efficiency of banana breeding programs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Marin DH, Romero RA, Guzman M, Sutton TB. Black Sigatoka: An Increasing Threat to Banana Cultivation. Plant Dis. 2003;87: 208–222. - PubMed
-
- Crous PW, Mourichon X. Mycosphaerella eumusae and its anamorph Pseudocercospora eumusae spp. nov.: causal agent of eumusae leaf spot disease of banana. Sydowia. 2002;54: 35–43.
-
- Carlier J, Lebrun MH, Zapater MF, Dubois C, Mourichon X. Genetic structure of the global population of banana black leaf streak fungus, Mycosphaerella fijiensis. Mol Ecol. 1996;5: 499–510. 10.1111/j.1365-294X.1996.tb00342.x - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

