Keratin 17 Is Induced in Oral Cancer and Facilitates Tumor Growth
- PMID: 27512993
- PMCID: PMC4981360
- DOI: 10.1371/journal.pone.0161163
Keratin 17 Is Induced in Oral Cancer and Facilitates Tumor Growth
Abstract
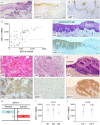
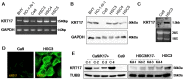
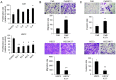
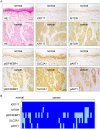
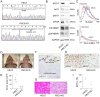
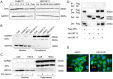
Keratin subtypes are selectively expressed depending on the cell type. They not only provide structural support, but regulate the metabolic processes and signaling pathways that control the growth of the epithelium. KRT17 (keratin 17) is induced in the regenerative epithelium and acts on diverse signaling pathways. Here, we demonstrate that KRT17 is invariably and permanently induced in oral squamous cell carcinoma (OSCC), as revealed by immunohistochemistry and cDNA microarray analysis. Two representative OSCC cell lines; KRT17-weakly expressing Ca9-22 and KRT17-highly expressing HSC3 were used to establish KRT17-overexpressing Ca9-22 and KRT17-knockdown HSC3 cells. Analysis of these cells revealed that KRT17 promoted cell proliferation and migration by stimulating the Akt/mTOR pathway. KRT17 also upregulated the expression of SLC2A1 (solute carrier family 2 member 1/Glut1) and glucose uptake. To further investigate the effect of KRT17 on tumorigenesis, KRT17-knockout HSC3 cells were established and were transplanted to the cephalic skin of nude mice. The tumors that developed from KRT17-knockout HSC3 cells had a lower Ki-67 labeling index and were significantly smaller compared to the controls. These results indicate that KRT17 stimulates the Akt/mTOR pathway and glucose uptake, thereby facilitating tumor growth. We could not confirm the relationship between KRT17 and SFN (stratifin) in the cells examined in this study. However, our study reinforces the concept that the cellular properties of cancer are regulated by a series of molecules similar to those found in wound healing. In OSCC, KRT17 acts as a pathogenic keratin that facilitates tumor growth through the stimulation of multiple signaling pathways, highlighting the importance of KRT17 as a multifunctional promoter of tumorigenesis.
Conflict of interest statement
Figures


References
-
- Quinlan RA, Schiller DL, Hatzfeld M, Achtstatter T, Moll R, Jorcano JL, et al. Patterns of expression and organization of cytokeratin intermediate filaments. Ann N Y Acad Sci. 1985;455:282–306. . - PubMed
-
- Fuchs E. Keratins and the skin. Annu Rev Cell Dev Biol. 1995;11:123–53. . - PubMed
-
- Mansbridge JN, Knapp AM. Changes in keratinocyte maturation during wound healing. J Invest Dermatol. 1987;89(3):253–63. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

