A placebo-controlled trial of simvastatin therapy in Smith-Lemli-Opitz syndrome
- PMID: 27513191
- PMCID: PMC5303568
- DOI: 10.1038/gim.2016.102
A placebo-controlled trial of simvastatin therapy in Smith-Lemli-Opitz syndrome
Abstract
Background: Smith-Lemli-Opitz syndrome (SLOS) is a multiple malformation/cognitive impairment syndrome characterized by the accumulation of 7-dehydrocholesterol, a precursor sterol of cholesterol. Simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor that crosses the blood-brain barrier, has been proposed for the treatment of SLOS based on in vitro and in vivo studies suggesting that simvastatin increases the expression of hypomorphic DHCR7 alleles.
Methods: Safety and efficacy of simvastatin therapy in 23 patients with mild to typical SLOS were evaluated in a randomized, double-blind, placebo-controlled trial. The crossover trial consisted of two 12-month treatment phases separated by a 2-month washout period.
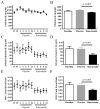
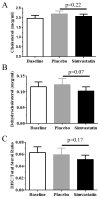
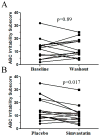
Results: No safety issues were identified in this study. Plasma dehydrocholesterol concentrations decreased significantly: 8.9 ± 8.4% on placebo to 6.1 ± 5.5% on simvastatin (P < 0.005); we observed a trend toward decreased cerebrospinal fluid dehydrocholesterol concentrations. A significant improvement (P = 0.017, paired t-test) was observed on the irritability subscale of the Aberrant Behavior Checklist-C when subjects were taking simvastatin.
Conclusion: This article reports what is, to our knowledge, the first randomized, placebo-controlled trial designed to test the safety and efficacy of simvastatin therapy in SLOS. Simvastatin seems to be relatively safe in patients with SLOS, improves the serum dehydrocholesterol-to-total sterol ratio, and significantly improves irritability symptoms in patients with mild to classic SLOS.Genet Med 19 3, 297-305.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

