Carbon Monoxide Improves Efficacy of Mesenchymal Stromal Cells During Sepsis by Production of Specialized Proresolving Lipid Mediators
- PMID: 27513357
- PMCID: PMC5113254
- DOI: 10.1097/CCM.0000000000001999
Carbon Monoxide Improves Efficacy of Mesenchymal Stromal Cells During Sepsis by Production of Specialized Proresolving Lipid Mediators
Abstract
Objectives: Mesenchymal stromal cells are being investigated as a cell-based therapy for a number of disease processes, with promising results in animal models of systemic inflammation and sepsis. Studies are ongoing to determine ways to further improve the therapeutic potential of mesenchymal stromal cells. A gas molecule that improves outcome in experimental sepsis is carbon monoxide. We hypothesized that preconditioning of mesenchymal stromal cells with carbon monoxide ex vivo would promote further therapeutic benefit when cells are administered in vivo after the onset of polymicrobial sepsis in mice.
Design: Animal study and primary cell culture.
Setting: Laboratory investigation.
Subjects: BALB/c mice.
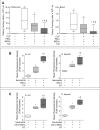
Interventions: Polymicrobial sepsis was induced by cecal ligation and puncture. Mesenchymal stromal cells, mesenchymal stromal cells-conditioned with carbon monoxide, fibroblasts, or fibroblasts-conditioned with carbon monoxide were delivered by tail vein injections to septic mice. The mice were assessed for survival, bacterial clearance, and the inflammatory response during sepsis in each of the groups. Mesenchymal stromal cells were also assessed for their ability to promote bacterial phagocytosis by neutrophils, the production of specialized proresolving lipid mediators, and their importance for mesenchymal stromal cells function using gene silencing.
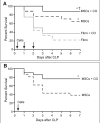
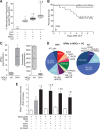
Measurements and main results: Ex vivo preconditioning with carbon monoxide allowed mesenchymal stromal cells to be administered later after the onset of sepsis (6 hr), and yet maintain their therapeutic effect with increased survival. Carbon monoxide preconditioned mesenchymal stromal cells were also able to alleviate organ injury, improve bacterial clearance, and promote the resolution of inflammation. Mesenchymal stromal cells exposed to carbon monoxide, with docosahexaenoic acid substrate, produced specialized proresolving lipid mediators, particularly D-series resolvins, which promoted survival. Silencing of lipoxygenase pathways (5-lipoxygenase and 12/15-lipoxygenase), which are important enzymes for specialized proresolving lipid mediator biosynthesis, resulted in a loss of therapeutic benefit bestowed on mesenchymal stromal cells by carbon monoxide.
Conclusions: Taken together, these data suggest that production of specialized proresolving lipid mediators contribute to improved mesenchymal stromal cell efficacy when exposed to carbon monoxide, resulting in an improved therapeutic response during sepsis.
Conflict of interest statement
Dr. Perrella received support for article research from the National Institutes of Health (NIH). His institution received funding from the NIH (National Heart, Lung, and Blood Institute [NHLBI]). Dr. Tsoyi received support for article research from the NIH. His institution received funding from the NIH. Drs. Dalli and Colas received support for article research from the NIH. Drs. Ghanta and Ith received support for article research from the NIH. Their institutions received funding from the NIH. Dr. Fredenburgh received support for article research from the NIH. Her institution received funding from the NHLBI. Drs. Baron and Choi received support for article research from the NIH. Dr. Serhan received support for article research from the NIH and disclosed other support (SAB member Corbus, SAB member Inflammation Foundation, SAB Solutex. He is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. He is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company [no current relationship but still hold founder stock]. His interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies). Dr. Liu received support for article research from the NIH and the AHA. Her institution received funding from the NIH and the AHA. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures


Comment in
-
Cellular Therapy for Sepsis: A New Hope?Crit Care Med. 2016 Dec;44(12):2296-2298. doi: 10.1097/CCM.0000000000002147. Crit Care Med. 2016. PMID: 27858821 Free PMC article. No abstract available.
-
Sepsis Therapies: Insights from Population Health to Cellular Therapies and Genomic Medicine.Am J Respir Crit Care Med. 2018 Dec 15;198(12):1570-1572. doi: 10.1164/rccm.201804-0782RR. Am J Respir Crit Care Med. 2018. PMID: 30277812 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

