The Effect of the Exon-3-Deleted Growth Hormone Receptor on Pegvisomant-Treated Acromegaly: A Systematic Review and Meta-Analysis
- PMID: 27513761
- PMCID: PMC5637298
- DOI: 10.1159/000448844
The Effect of the Exon-3-Deleted Growth Hormone Receptor on Pegvisomant-Treated Acromegaly: A Systematic Review and Meta-Analysis
Abstract
Background: The common exon 3 deletion polymorphism of the growth hormone receptor (d3-GHR) is associated with disease severity in acromegaly patients. The GHR antagonist pegvisomant (PEGV) is highly effective in treating severe acromegaly. Response to PEGV treatment seems to be influenced by d3-GHR and appears to be more responsive to PEGV, although available results remain conflicting.
Objective: To assess the influence of d3-GHR on the responsiveness of acromegaly patients to PEGV by compiling the evidence derived from the largest available studies.
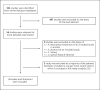
Design: A systematic review of the literature identified three published studies and one conference abstract. Acromegaly patients (n = 324, 49.7% d3-GHR carriers) were treated with either PEGV monotherapy or PEGV combined with long-acting somatostatin analogues and/or cabergoline. A meta-analysis of raw data from these studies was performed.
Results: No significant effect of the d3-GHR was observed while bringing insulin-like growth factor I (IGF-I) levels below the upper limit of normal with PEGV, which was defined as the lowest IGF-I level during PEGV treatment (mean difference: -2.3%; 95% CI: -6.5 to 1.8, p = 0.270). The PEGV dose required to achieve the lowest IGF-I levels was also not significantly influenced by individuals carrying d3-GHR (mean difference: 4.1 mg weekly; 95% CI: -5.1 to 13.2, p = 0.385). For both outcomes, separate analysis of PEGV monotherapy and combination treatment gave similar results.
Conclusion: Our findings suggest that the d3-GHR polymorphism has no effect on biochemical disease control in acromegaly, as it is not of added value for either the prediction of PEGV responsiveness or the determination of the required PEGV dose.
Keywords: Acromegaly; Deletion of exon 3; Growth hormone receptor; Meta-analysis; Pegvisomant; Polymorphism.
© 2016 The Author(s) Published by S. Karger AG, Basel.
Figures


References
-
- Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355:2558–2573. - PubMed
-
- Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159:89–95. - PubMed
-
- Bates PR, Carson MN, Trainer PJ, Wass JA, UK National Acromegaly Register Study Group Wide variation in surgical outcomes for acromegaly in the UK. Clin Endocrinol (Oxf) 2008;68:136–142. - PubMed
-
- Holdaway IM. Treatment of acromegaly. Horm Res. 2004;62((suppl 3)):79–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

