Structural diversity of halocarbonyl molybdenum and tungsten PNP pincer complexes through ligand modifications
- PMID: 27513832
- PMCID: PMC5048400
- DOI: 10.1039/c6dt02251k
Structural diversity of halocarbonyl molybdenum and tungsten PNP pincer complexes through ligand modifications
Abstract
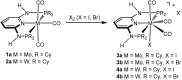
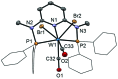
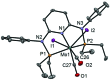
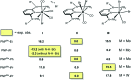
This work presents a comparative study of a series of halocarbonyl Mo(ii) and W(ii) complexes of the types [M(PNP)(CO)3X]X and [M(PNP)(CO)2X2] (M = Mo, W; X = I, Br), featuring PNP pincer ligands based on a 2,6-diaminopyridine scaffold. The complexes were prepared and fully characterized. The syntheses of these complexes were accomplished by treatment of [M(PNP)(CO)3] with stoichiometric amounts of I2 and Br2, respectively. The modification of the 2,6-diaminopyridine scaffold by introducing NMe and NPh instead of NH spacers with concomitant modification of the phosphine moieties changed the steric and electronic properties of the PNP ligand significantly. While in the case of NH linkers exclusively cationic seven-coordinate complexes of the type [M(PNP)(CO)3X](+) were obtained with NMe and NPh spacers neutral seven-coordinate complexes of the type [M(PNP)(CO)2X2] were afforded. In the case of the latter, when the reaction is performed in the presence of CO also [M(PNP)(CO)3X](+) complexes are formed which slowly lose CO to give [M(PNP)(CO)2X2]. The halocarbonyl tungsten chemistry parallels that of molybdenum. The only exception is molybdenum in conjunction with the PNP(Me)-iPr ligand, where the coordinatively unsaturated complex [Mo(PNP(Me)-iPr)(CO)X2] is formed. DFT mechanistic studies reveal that the seven-coordinate complexes should be the thermodynamic as well as the kinetic products. Since [Mo(PNP(Me)-iPr)(CO)X2] is the observed product it suggests that the reaction follows an alternative path. Structures of representative complexes were determined by X-ray single crystal analyses.
Figures
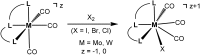











Similar articles
-
Synthesis and reactivity of coordinatively unsaturated halocarbonyl molybdenum PNP pincer complexes.Dalton Trans. 2014 Oct 21;43(39):14669-79. doi: 10.1039/c4dt01932f. Dalton Trans. 2014. PMID: 25142749
-
A complete series of halocarbonyl molybdenum PNP pincer complexes - Unexpected differences between NH and NMe spacers.J Organomet Chem. 2014 Jun 15;760(100):74-83. doi: 10.1016/j.jorganchem.2013.12.018. J Organomet Chem. 2014. PMID: 24940004 Free PMC article.
-
Synthesis and Characterization of Hydrido Carbonyl Molybdenum and Tungsten PNP Pincer Complexes.Organometallics. 2013 May 24;32(10):3042-3052. doi: 10.1021/om400254k. Epub 2013 May 7. Organometallics. 2013. PMID: 23794778 Free PMC article.
-
Formation of Mono Oxo Molybdenum(IV) PNP Pincer Complexes: Interplay between Water and Molecular Oxygen.Eur J Inorg Chem. 2018 Feb 21;2018(7):876-884. doi: 10.1002/ejic.201701413. Epub 2018 Feb 12. Eur J Inorg Chem. 2018. PMID: 31057330 Free PMC article.
-
Synthesis and reactivity of TADDOL-based chiral Fe(II) PNP pincer complexes-solution equilibria between κ(2)P,N- and κ(3)P,N,P-bound PNP pincer ligands.Dalton Trans. 2015 Aug 7;44(29):13071-86. doi: 10.1039/c5dt00832h. Dalton Trans. 2015. PMID: 26104487
Cited by
-
Synthesis and characterization of TADDOL-based chiral group six PNP pincer tricarbonyl complexes.Monatsh Chem. 2019;150(1):103-109. doi: 10.1007/s00706-018-2281-0. Epub 2018 Oct 20. Monatsh Chem. 2019. PMID: 30662092 Free PMC article.
References
-
- Dilsky S. J. Organomet. Chem. 2007;692:2887.
- Song L.-C., Zhang L.-Y., Hu Q.-M., Huang X.-Y. Inorg. Chim. Acta. 1995;230:127.
- Curtis M. D., Shiu K. Inorg. Chem. 1985;24:1213.
- Schwarz C. L., Bullock R. M., Creutz C. J. Am. Chem. Soc. 1991;113:1225.
- Chaudhuri P., Wieghardt K., Tsai Y. H., Krüger C. Inorg. Chem. 1984;23:427.
- Baker K. P. Adv. Organomet. Chem. 1996;40:45.
- Edwards P. G., Fleming J. S., Liyanage S. S. Inorg. Chem. 1996;35:4563.
- Coles S. J., Edwards P. G., Fleming J. S., Hursthouse M. B. J. Chem. Soc., Dalton Trans. 1995:4091.
- Planinic P., Meider H. Polyhedron. 1990;9:1099.
-
- Wingard L. A., White P. S., Templeton J. L. Dalton Trans. 2012;41:11438. - PubMed
-
- Benito-Garagorri D., Becker E., Wiedermann J., Lackner W., Pollak M., Mereiter K., Kisala J., Kirchner K. Organometallics. 2006;25:1900.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

