Silica-Triggered Autoimmunity in Lupus-Prone Mice Blocked by Docosahexaenoic Acid Consumption
- PMID: 27513935
- PMCID: PMC4981380
- DOI: 10.1371/journal.pone.0160622
Silica-Triggered Autoimmunity in Lupus-Prone Mice Blocked by Docosahexaenoic Acid Consumption
Erratum in
-
Correction: Silica-Triggered Autoimmunity in Lupus-Prone Mice Blocked by Docosahexaenoic Acid Consumption.PLoS One. 2017 Feb 6;12(2):e0171877. doi: 10.1371/journal.pone.0171877. eCollection 2017. PLoS One. 2017. PMID: 28166264 Free PMC article.
-
Correction: Silica-Triggered Autoimmunity in Lupus-Prone Mice Blocked by Docosahexaenoic Acid Consumption.PLoS One. 2019 Jul 24;14(7):e0220469. doi: 10.1371/journal.pone.0220469. eCollection 2019. PLoS One. 2019. PMID: 31339949 Free PMC article.
Abstract
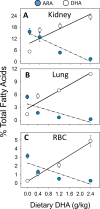
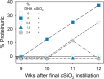
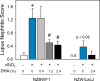
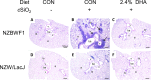
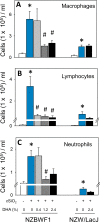
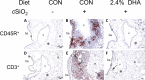
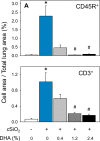
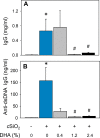
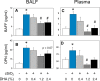
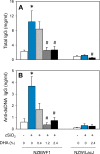
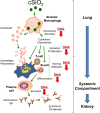
Occupational exposure to respirable crystalline silica (cSiO2, quartz) is etiologically linked to systemic lupus erythematosus (lupus) and other human autoimmune diseases (ADs). In the female NZBWF1 mouse, a widely used animal model that is genetically prone to lupus, short-term repeated intranasal exposure to cSiO2 triggers premature initiation of autoimmune responses in the lungs and kidneys. In contrast to cSiO2's triggering action, consumption of the ω-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) prevents spontaneous onset of autoimmunity in this mouse strain. The aim of this study was to test the hypothesis that consumption of DHA will prevent cSiO2-triggered autoimmunity in the female NZBWF1 mouse. Mice (6 wk old) were fed isocaloric AIN-93G diets containing 0.0, 0.4, 1.2 or 2.4% DHA. Two wk after initiating feeding, mice were intranasally instilled with 1 mg cSiO2 once per wk for 4 wk and maintained on experimental diets for an additional 12 wk. Mice were then sacrificed and the lung, blood and kidney assessed for markers of inflammation and autoimmunity. DHA was incorporated into lung, red blood cells and kidney from diet in a concentration-dependent fashion. Dietary DHA dose-dependently suppressed cSiO2-triggered perivascular leukocyte infiltration and ectopic lymphoid tissue neogenesis in the lung. DHA consumption concurrently inhibited cSiO2-driven elevation of proinflammatory cytokines, B-cell proliferation factors, IgG and anti-dsDNA Ig in both bronchoalveolar lavage fluid and plasma. DHA's prophylactic effects were further mirrored in reduced proteinuria and glomerulonephritis in cSiO2-treated mice. Taken together, these results reveal that DHA consumption suppresses cSiO2 triggering of autoimmunity in female NZBWF1 mice as manifested in the lung, blood and kidney. Our findings provide novel insight into how dietary modulation of the lipidome might be used to prevent or delay triggering of AD by cSiO2. Such knowledge opens the possibility of developing practical, low-cost preventative strategies to reduce the risk of initiating AD and subsequent flaring in cSiO2-exposed individuals. Additional research in this model is required to establish the mechanisms by which DHA suppresses cSiO2-induced autoimmunity and to ascertain unique lipidome signatures predictive of susceptibility to cSiO2-triggered AD.
Conflict of interest statement
Figures


References
-
- NIEHS. Autoimmune diseases. 2012. https://www.niehs.nih.gov/health/materials/autoimmune_diseases_508.pdf. Accessed 7/21/2016.
-
- OSHA. OSHA's final rule to protect workers from exposure to respirable crystalline silica. 2016. https://www.osha.gov/silica/. Accessed 7/21/2016.
-
- Parks CG, Cooper GS, Nylander-French LA, Sanderson WT, Dement JM, Cohen PL et al. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus—A population-based, case-control study in the southeastern United States. Arthr Rheum. 2002;46(7):1840–1850. 10.1002/art.10368 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

