Locus coeruleus volume and cell population changes during Alzheimer's disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery
- PMID: 27513978
- PMCID: PMC5298942
- DOI: 10.1016/j.jalz.2016.06.2362
Locus coeruleus volume and cell population changes during Alzheimer's disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery
Abstract
Introduction: Alzheimer's disease (AD) progression follows a specific spreading pattern, emphasizing the need to characterize those brain areas that degenerate first. The brainstem's locus coeruleus (LC) is the first area to develop neurofibrillary changes (neurofibrillary tangles [NFTs]).
Methods: The methods include unbiased stereological analyses in human brainstems to estimate LC volume and neuronal population in controls and individuals across all AD stages.
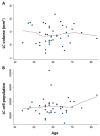
Results: As the Braak stage increases by 1 unit, the LC volume decreases by 8.4%. Neuronal loss started only midway through AD progression. Age-related changes spare the LC.
Discussion: The long gap between NFT accumulation and neuronal loss suggests that a second trigger may be necessary to induce neuronal death in AD. Imaging studies should determine whether LC volumetry can replicate the stage-wise atrophy observed here and how these changes are specific to AD. LC volumetry may develop into a screening biomarker for selecting high-yield candidates to undergo expensive and less accessible positron emission tomography scans and to monitor AD progression from presymptomatic stages.
Keywords: Alzheimer's disease; Brainstem; Human; Locus coeruleus; Neurofibrillary tangles; Neuron counts; Postmortem; Unbiased stereology; Volumetry.
Copyright © 2016 the Alzheimer's Association. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Hurd MD, Martorell P, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;369:489–490. - PubMed
-
- Korczyn AD. Why have we failed to cure Alzheimer’s disease? J Alzheimers Dis. 2012;29:275–282. - PubMed
-
- Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. - PubMed
-
- Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E, da Silva CG, Guimaraes DM, Szczupak D, Parente-Bruno DR, Carvalho LR, Polichiso L, Gomes BV, Oliveira LM, Rodriguez RD, Leite RE, Ferretti-Rebustini RE, Jacob-Filho W, Pasqualucci CA, Grinberg LT, Lent R. Cell number changes in Alzheimer’s disease relate to dementia not to plaques and tangles. Brain. 2013;136:3738–3752. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

