Long-term survival and regeneration of neuronal and vasculature cells inside the core region after ischemic stroke in adult mice
- PMID: 27514013
- PMCID: PMC5303567
- DOI: 10.1111/bpa.12425
Long-term survival and regeneration of neuronal and vasculature cells inside the core region after ischemic stroke in adult mice
Abstract
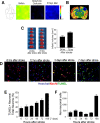
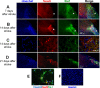
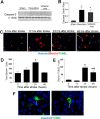
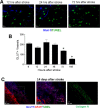
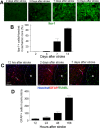
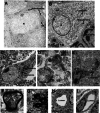
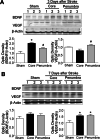
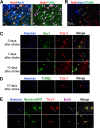
Focal cerebral ischemia results in an ischemic core surrounded by the peri-infarct region (penumbra). Most research attention has been focused on penumbra while the pattern of cell fates inside the ischemic core is poorly defined. In the present investigation, we tested the hypothesis that, inside the ischemic core, some neuronal and vascular cells could survive the initial ischemic insult while regenerative niches might exist many days after stroke in the adult brain. Adult mice were subjected to focal cerebral ischemia induced by permanent occlusion of distal branches of the middle cerebral artery (MCA) plus transient ligations of bilateral common carotid artery (CCA). The ischemic insult uniformly reduced the local cerebral blood flow (LCBF) by 90%. Massive cell death occurred due to multiple mechanisms and a significant infarction was cultivated in the ischemic cortex 24 h later. Nevertheless, normal or even higher levels of brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) persistently remained in the core tissue, some NeuN-positive and Glut-1/College IV-positive cells with intact ultrastructural features resided in the core 7-14 days post stroke. BrdU-positive but TUNEL-negative neuronal and endothelial cells were detected in the core where extensive extracellular matrix infrastructure developed. Meanwhile, GFAP-positive astrocytes accumulated in the penumbra and Iba-1-positive microglial/macrophages invaded the core several days after stroke. The long term survival of neuronal and vascular cells inside the ischemic core was also seen after a severe ischemic stroke induced by permanent embolic occlusion of the MCA. We demonstrate that a therapeutic intervention of pharmacological hypothermia could save neurons/endothelial cells inside the core. These data suggest that the ischemic core is an actively regulated brain region with residual and newly formed viable neuronal and vascular cells acutely and chronically after at least some types of ischemic strokes.
Keywords: cell survival; ischemic core; neurotrophic factors; neurovasculature; regeneration.
© 2016 International Society of Neuropathology.
Figures


Similar articles
-
Gonadal steroids block the calpain-1-dependent intrinsic pathway of apoptosis in an experimental rat stroke model.Neurol Res. 2017 Jan;39(1):54-64. doi: 10.1080/01616412.2016.1250459. Epub 2016 Nov 11. Neurol Res. 2017. PMID: 27832728
-
Pyruvate Kinase M2 Increases Angiogenesis, Neurogenesis, and Functional Recovery Mediated by Upregulation of STAT3 and Focal Adhesion Kinase Activities After Ischemic Stroke in Adult Mice.Neurotherapeutics. 2018 Jul;15(3):770-784. doi: 10.1007/s13311-018-0635-2. Neurotherapeutics. 2018. PMID: 29869055 Free PMC article.
-
VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration.J Neurosci Res. 2007 Mar;85(4):740-7. doi: 10.1002/jnr.21169. J Neurosci Res. 2007. PMID: 17243175
-
Regional cerebral blood flow after occlusion of the middle cerebral artery.Acta Neurol Scand. 1986 Apr;73(4):321-37. doi: 10.1111/j.1600-0404.1986.tb03286.x. Acta Neurol Scand. 1986. PMID: 3524091 Review.
-
Pathophysiology and treatment of cerebral ischemia.J Med Invest. 1998 Aug;45(1-4):57-70. J Med Invest. 1998. PMID: 9864965 Review.
Cited by
-
Potential Variables for Improved Reproducibility of Neuronal Cell Grafts at Stroke Sites.Cells. 2022 May 17;11(10):1656. doi: 10.3390/cells11101656. Cells. 2022. PMID: 35626693 Free PMC article.
-
Delayed PARP-1 Inhibition Alleviates Post-stroke Inflammation in Male Versus Female Mice: Differences and Similarities.Front Cell Neurosci. 2020 Apr 3;14:77. doi: 10.3389/fncel.2020.00077. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32317937 Free PMC article.
-
Cerebral ischemia and neuroregeneration.Neural Regen Res. 2018 Mar;13(3):373-385. doi: 10.4103/1673-5374.228711. Neural Regen Res. 2018. PMID: 29623912 Free PMC article. Review.
-
Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke.Prog Neurobiol. 2017 Oct;157:49-78. doi: 10.1016/j.pneurobio.2017.03.003. Epub 2017 Mar 18. Prog Neurobiol. 2017. PMID: 28322920 Free PMC article. Review.
-
Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice.Neurobiol Dis. 2016 Dec;96:248-260. doi: 10.1016/j.nbd.2016.09.013. Epub 2016 Sep 19. Neurobiol Dis. 2016. PMID: 27659107 Free PMC article.
References
-
- Aronowski J, Cho KH, Strong R, Grotta JC (1999) Neurofilament proteolysis after focal ischemia; when do cells die after experimental stroke? J Cereb Blood Flow Metab 19:652–660. - PubMed
-
- Barber PA, Davis SM, Infeld B, Baird AE, Donnan GA, Jolley D, Lichtenstein M (1998) Spontaneous reperfusion after ischemic stroke is associated with improved outcome. Stroke 29:2522–2528. - PubMed
-
- Barone FC (2009) Ischemic stroke intervention requires mixed cellular protection of the penumbra. Curr Opin Investig Drugs 10:220–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

