Regulatory roles of interferon-inducible protein 204 on differentiation and vasculogenic activity of endothelial progenitor cells
- PMID: 27514835
- PMCID: PMC4981987
- DOI: 10.1186/s13287-016-0365-5
Regulatory roles of interferon-inducible protein 204 on differentiation and vasculogenic activity of endothelial progenitor cells
Abstract
Background: Endothelial progenitor cells (EPCs) have shown great potential in angiogenesis either by their differentiation into endothelial cells or by secretion of angiogenic factors. Interferon-inducible protein 204 (Ifi204) has been reported to participate in the regulation of cell growth and differentiation. However, its role in differentiation of EPCs remains unknown. We proposed that Ifi204 could modulate the differentiation and regenerative abilities of EPCs.
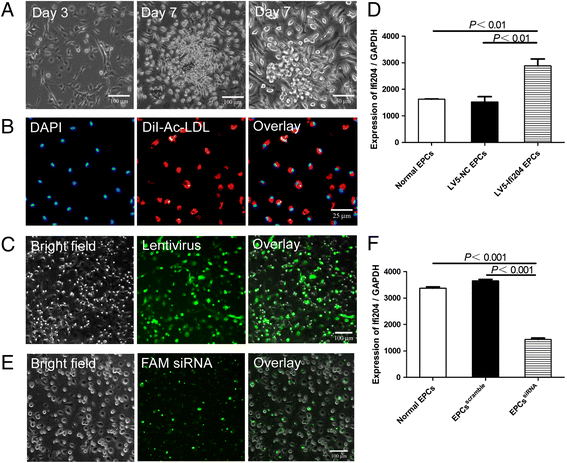
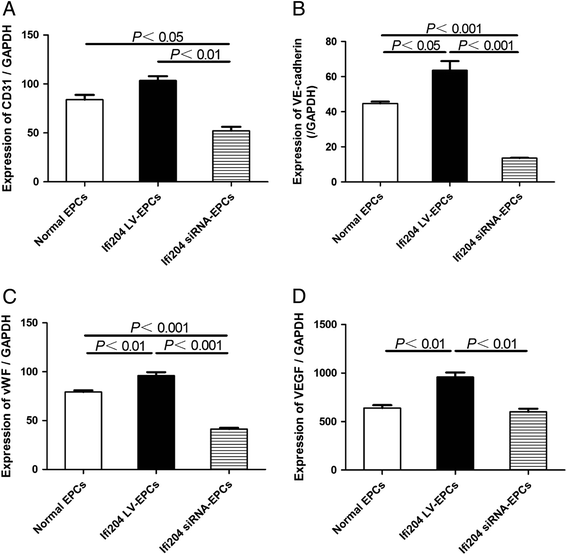
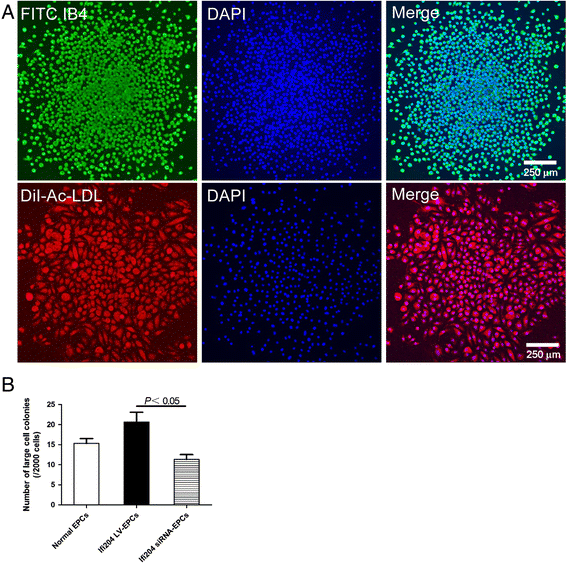
Methods: Ifi204-expressing lentivirus and Ifi204 siRNA were introduced into EPCs to overexpress and suppress the expression of Ifi204. Using fluorescence-activated cell sorting, immunocytochemistry, and quantitative PCR, endothelial markers including CD31, VE-cadherin, and vWF were detected in the modified EPCs. An in-vitro incorporation assay and a colony-forming assay were also performed.
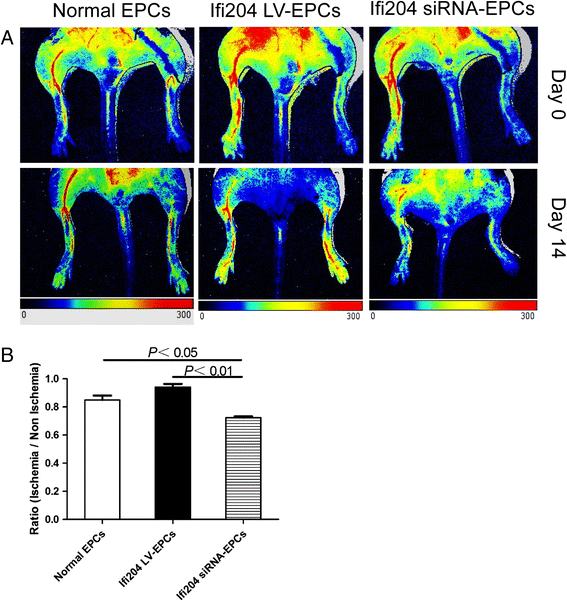
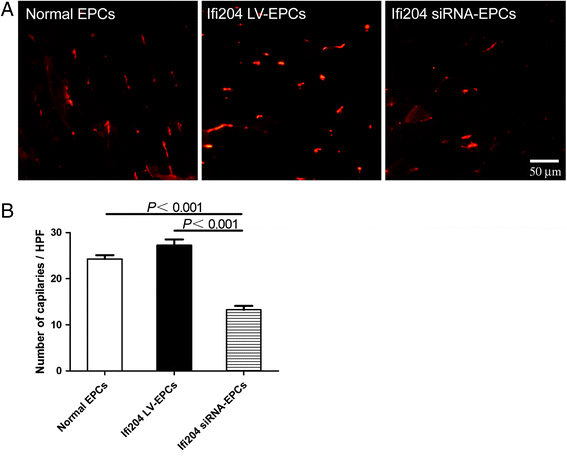
Results: Evidence showed that Ifi204 inhibition decreased the endothelial differentiation and vasculogenic activities of EPCs in vitro. In mice with hindlimb ischemia, downregulation of Ifi204 in EPCs, which was tracked by our newly synthesized nanofluorogen, impaired neovascularization, with a corresponding reduction in hindlimb blood reperfusion by postoperative day 14.
Conclusions: Ifi204 is required for EPC differentiation and neovascularization in vitro and in vivo. The regulatory roles of Ifi204 in EPC differentiation may benefit the clinical therapy of ischemic vascular diseases.
Keywords: Endothelial differentiation; Endothelial progenitor cells; Hindlimb ischemia; Ifi204; Vasculogenesis.
Figures



Similar articles
-
Identification of mouse colony-forming endothelial progenitor cells for postnatal neovascularization: a novel insight highlighted by new mouse colony-forming assay.Stem Cell Res Ther. 2013 Feb 28;4(1):20. doi: 10.1186/scrt168. Stem Cell Res Ther. 2013. PMID: 23448126 Free PMC article.
-
Angiogenic potential of early and late outgrowth endothelial progenitor cells is dependent on the time of emergence.Int J Cardiol. 2015;186:305-14. doi: 10.1016/j.ijcard.2015.03.166. Epub 2015 Mar 17. Int J Cardiol. 2015. PMID: 25838182
-
Statins, HMG-CoA Reductase Inhibitors, Improve Neovascularization by Increasing the Expression Density of CXCR4 in Endothelial Progenitor Cells.PLoS One. 2015 Aug 26;10(8):e0136405. doi: 10.1371/journal.pone.0136405. eCollection 2015. PLoS One. 2015. PMID: 26309120 Free PMC article.
-
Endothelial Progenitor Cells: A Review of Molecular Mechanisms in the Pathogenesis and Endovascular Treatment of Intracranial Aneurysms.Neuromolecular Med. 2024 Jun 17;26(1):25. doi: 10.1007/s12017-024-08791-4. Neuromolecular Med. 2024. PMID: 38886284 Review.
-
Biologic properties of endothelial progenitor cells and their potential for cell therapy.Prog Cardiovasc Dis. 2007 May-Jun;49(6):421-9. doi: 10.1016/j.pcad.2007.02.004. Prog Cardiovasc Dis. 2007. PMID: 17498522 Free PMC article. Review.
Cited by
-
Sonic hedgehog promotes endothelial differentiation of bone marrow mesenchymal stem cells via VEGF-D.J Thorac Dis. 2018 Sep;10(9):5476-5488. doi: 10.21037/jtd.2018.09.50. J Thorac Dis. 2018. PMID: 30416797 Free PMC article.
-
Structural mechanism of DNA recognition by the p204 HIN domain.Nucleic Acids Res. 2021 Mar 18;49(5):2959-2972. doi: 10.1093/nar/gkab076. Nucleic Acids Res. 2021. PMID: 33619523 Free PMC article.
-
Endothelial progenitor cell-derived exosomes, loaded with miR-126, promoted deep vein thrombosis resolution and recanalization.Stem Cell Res Ther. 2018 Aug 23;9(1):223. doi: 10.1186/s13287-018-0952-8. Stem Cell Res Ther. 2018. Retraction in: Stem Cell Res Ther. 2019 Jun 11;10(1):162. doi: 10.1186/s13287-019-1264-3. PMID: 30139377 Free PMC article. Retracted.
-
Curcumin induces therapeutic angiogenesis in a diabetic mouse hindlimb ischemia model via modulating the function of endothelial progenitor cells.Stem Cell Res Ther. 2017 Aug 3;8(1):182. doi: 10.1186/s13287-017-0636-9. Stem Cell Res Ther. 2017. PMID: 28774328 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

