Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer's disease
- PMID: 27514990
- PMCID: PMC4981845
- DOI: 10.1038/srep31450
Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer's disease
Abstract
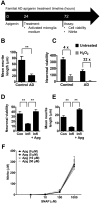
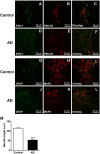
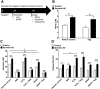
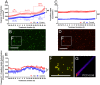
Alzheimer's disease (AD) is one of the most prevalent neurodegenerative diseases, yet current therapeutic treatments are inadequate due to a complex disease pathogenesis. The plant polyphenol apigenin has been shown to have anti-inflammatory and neuroprotective properties in a number of cell and animal models; however a comprehensive assessment has not been performed in a human model of AD. Here we have used a human induced pluripotent stem cell (iPSC) model of familial and sporadic AD, in addition to healthy controls, to assess the neuroprotective activity of apigenin. The iPSC-derived AD neurons demonstrated a hyper-excitable calcium signalling phenotype, elevated levels of nitrite, increased cytotoxicity and apoptosis, reduced neurite length and increased susceptibility to inflammatory stress challenge from activated murine microglia, in comparison to control neurons. We identified that apigenin has potent anti-inflammatory properties with the ability to protect neurites and cell viability by promoting a global down-regulation of cytokine and nitric oxide (NO) release in inflammatory cells. In addition, we show that apigenin is able to protect iPSC-derived AD neurons via multiple means by reducing the frequency of spontaneous Ca(2+) signals and significantly reducing caspase-3/7 mediated apoptosis. These data demonstrate the broad neuroprotective action of apigenin against AD pathogenesis in a human disease model.
Figures

References
-
- Poirier J. et al. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet 342, 697 (1993). - PubMed
-
- Haass C. et al. The Swedish mutation causes early-onset Alzheimer’s disease by [beta]-secretase cleavage within the secretory pathway. Nat Med 1, 1291–1296 (1995). - PubMed
-
- Larson J., Lynch G., Games D. & Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res . 840, 23–35 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

