Mechanisms of Hsp90 regulation
- PMID: 27515256
- PMCID: PMC4980810
- DOI: 10.1042/BCJ20160005
Mechanisms of Hsp90 regulation
Abstract
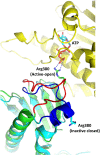
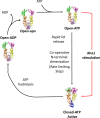
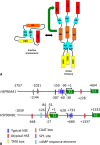
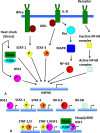
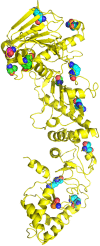
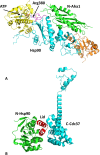
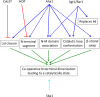
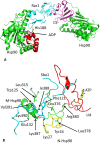
Heat shock protein 90 (Hsp90) is a molecular chaperone that is involved in the activation of disparate client proteins. This implicates Hsp90 in diverse biological processes that require a variety of co-ordinated regulatory mechanisms to control its activity. Perhaps the most important regulator is heat shock factor 1 (HSF1), which is primarily responsible for upregulating Hsp90 by binding heat shock elements (HSEs) within Hsp90 promoters. HSF1 is itself subject to a variety of regulatory processes and can directly respond to stress. HSF1 also interacts with a variety of transcriptional factors that help integrate biological signals, which in turn regulate Hsp90 appropriately. Because of the diverse clientele of Hsp90 a whole variety of co-chaperones also regulate its activity and some are directly responsible for delivery of client protein. Consequently, co-chaperones themselves, like Hsp90, are also subject to regulatory mechanisms such as post translational modification. This review, looks at the many different levels by which Hsp90 activity is ultimately regulated.
Keywords: HSF1; Hsp90; chaperones; co-chaperones; heat-shock response; post-translational modification.
© 2016 The Author(s).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

