Tumor-associated stromal cells as key contributors to the tumor microenvironment
- PMID: 27515302
- PMCID: PMC4982339
- DOI: 10.1186/s13058-016-0740-2
Tumor-associated stromal cells as key contributors to the tumor microenvironment
Abstract
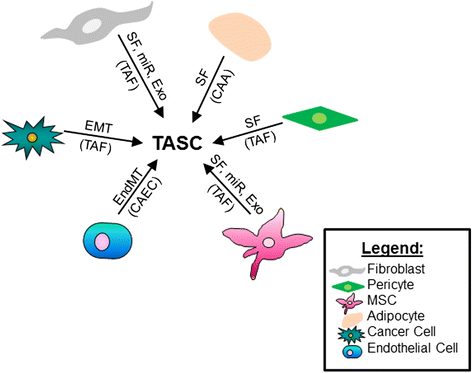
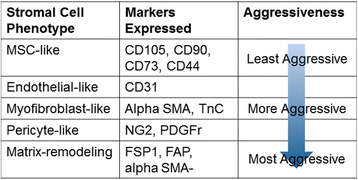
The tumor microenvironment is a heterogeneous population of cells consisting of the tumor bulk plus supporting cells. It is becoming increasingly evident that these supporting cells are recruited by cancer cells from nearby endogenous host stroma and promote events such as tumor angiogenesis, proliferation, invasion, and metastasis, as well as mediate mechanisms of therapeutic resistance. In addition, recruited stromal cells range in type and include vascular endothelial cells, pericytes, adipocytes, fibroblasts, and bone-marrow mesenchymal stromal cells. During normal wound healing and inflammatory processes, local stromal cells change their phenotype to become that of reactive stroma. Under certain conditions, however, tumor cells can co-opt these reactive stromal cells and further transition them into tumor-associated stromal cells (TASCs). These TASCs express higher levels of proteins, including alpha-smooth muscle actin, fibroblast activating protein, and matrix metalloproteinases, compared with their normal, non-reactive counterparts. TASCs are also known to secrete many pro-tumorigenic factors, including IL-6, IL-8, stromal-derived factor-1 alpha, vascular endothelial growth factor, tenascin-C, and matrix metalloproteinases, among others, which recruit additional tumor and pro-tumorigenic cells to the developing microenvironment. Here, we review the current literature pertaining to the origins of recruited host stroma, contributions toward tumor progression, tumor-associated stromal cells, and mechanisms of crosstalk between endogenous host stroma and tumor cells.
Keywords: Alpha-smooth muscle actin; Cancer-associated fibroblast; Exosome; IL-6; MCP-1; Mesenchymal stem cell; Myofibroblast; Stroma; Tumor microenvironment; Tumor-associated fibroblast; Tumor-associated stroma; microRNA.
Figures
References
-
- Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–14. doi: 10.1073/pnas.1013805107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

