A whole blood model of thrombocytopenia that controls platelet count and hematocrit
- PMID: 27515424
- PMCID: PMC6055520
- DOI: 10.1007/s00277-016-2777-9
A whole blood model of thrombocytopenia that controls platelet count and hematocrit
Abstract
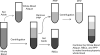
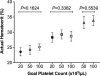
In patients with thrombocytopenia, it can be difficult to predict a patient's bleeding risk based on platelet count alone. Platelet reactivity may provide additional information; however, current clinical assays cannot reliably assess platelet function in the setting of thrombocytopenia. New methods to study platelet reactivity in thrombocytopenic samples are needed. In this study, we sought to develop a laboratory model of thrombocytopenia using blood from healthy subjects that preserves the whole blood environment and reproducibly produces samples with a specific platelet count and hematocrit. We compared the activation state of unstimulated and agonist-stimulated platelets in thrombocytopenic samples derived from this method with normocytic controls. Whole blood was diluted with autologous red blood cell concentrate and platelet-poor plasma, which were obtained via centrifugation, in specific ratios to attain a final sample with a predetermined platelet count and hematocrit. P-selectin exposure and GPIIbIIIa activation in unstimulated platelets and platelets stimulated with collagen-related peptide (CRP) or adenosine diphosphate (ADP) in thrombocytopenic samples and the normocytic control from which they were derived were quantified by flow cytometry. Our methodology reliably produced thrombocytopenic samples with a platelet count ≤50,000/μL and an accurately and precisely controlled hematocrit. P-selectin exposure and GPIIbIIIa activation on unstimulated platelets or on ADP- or CRP-stimulated platelets did not differ in thrombocytopenic samples compared to normocytic controls. We describe a new method for creating thrombocytopenic blood that can be used to better understand the contributions of platelet number and function to hemostasis.
Keywords: Blood platelets; Flow cytometry; Platelet activation; Platelet aggregation; Thrombocytopenia.
Conflict of interest statement
Figures



Similar articles
-
Measurement of platelet aggregation, independently of patient platelet count: a flow-cytometric approach.J Thromb Haemost. 2017 Jun;15(6):1191-1202. doi: 10.1111/jth.13675. Epub 2017 May 3. J Thromb Haemost. 2017. PMID: 28296243
-
Thrombocytopenia model with minimal manipulation of blood cells allowing whole blood assessment of platelet function.Platelets. 2016 Jun;27(4):295-300. doi: 10.3109/09537104.2015.1095873. Epub 2015 Nov 10. Platelets. 2016. PMID: 26555800
-
Change of platelet activation markers using flow cytometry in patients with hematology/oncology disorders after transfusion.Platelets. 2008 Aug;19(5):328-34. doi: 10.1080/09537100802129867. Platelets. 2008. PMID: 18791938
-
An overview of platelet indices and methods for evaluating platelet function in thrombocytopenic patients.Eur J Haematol. 2014;92(5):367-76. doi: 10.1111/ejh.12262. Epub 2014 Mar 12. Eur J Haematol. 2014. PMID: 24400878 Review.
-
The role of platelets in bleeding in patients with thrombocytopenia and hematological disease.Clin Chem Lab Med. 2019 Nov 26;57(12):1808-1817. doi: 10.1515/cclm-2019-0380. Clin Chem Lab Med. 2019. PMID: 31465290 Review.
Cited by
-
Platelet Function Changes during Neonatal Cardiopulmonary Bypass Surgery: Mechanistic Basis and Lack of Correlation with Excessive Bleeding.Thromb Haemost. 2020 Jan;120(1):94-106. doi: 10.1055/s-0039-1700517. Epub 2019 Nov 21. Thromb Haemost. 2020. PMID: 31752040 Free PMC article.
-
Assay validity of point-of-care platelet function tests in thrombocytopenic blood samples.Biochem Med (Zagreb). 2022 Jun 15;32(2):020713. doi: 10.11613/BM.2022.020713. Biochem Med (Zagreb). 2022. PMID: 35799989 Free PMC article.
-
Conditional CRISPR-mediated deletion of Lyn kinase enhances differentiation and function of iPSC-derived megakaryocytes.J Thromb Haemost. 2022 Jan;20(1):182-195. doi: 10.1111/jth.15546. Epub 2021 Oct 17. J Thromb Haemost. 2022. PMID: 34624170 Free PMC article.
-
Diacylglycerol kinase ζ is a negative regulator of GPVI-mediated platelet activation.Blood Adv. 2019 Apr 9;3(7):1154-1166. doi: 10.1182/bloodadvances.2018026328. Blood Adv. 2019. PMID: 30967391 Free PMC article.
-
Platelet count reduction during in vitro membrane oxygenation affects platelet activation, neutrophil extracellular trap formation and clot stability, but does not prevent clotting.Perfusion. 2022 Mar;37(2):134-143. doi: 10.1177/0267659121989231. Epub 2021 Jan 21. Perfusion. 2022. PMID: 33475044 Free PMC article.
References
-
- Stanworth SJ, Estcourt LJ, Powter G, Kahan BC, Dyer C, Choo L, Bakrania L, Llewelyn C, Littlewood T, Soutar R, Norfolk D, Copplestone A, Smith N, Kerr P, Jones G, Raj K, Westerman DA, Szer J, Jackson N, Bardy PG, Plews D, Lyons S, Bielby L, Wood EM, Murphy MF Investigators T. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368(19):1771–1780. doi: 10.1056/NEJMoa1212772. - DOI - PubMed
-
- Wandt H, Schaefer-Eckart K, Wendelin K, Pilz B, Wilhelm M, Thalheimer M, Mahlknecht U, Ho A, Schaich M, Kramer M, Kaufmann M, Leimer L, Schwerdtfeger R, Conradi R, Dolken G, Klenner A, Hanel M, Herbst R, Junghanss C, Ehninger G, Study Alliance L. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet. 2012;380(9850):1309–1316. doi: 10.1016/S0140-6736(12)60689-8. - DOI - PubMed
-
- Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, Gernsheimer TB, Ness PM, Brecher ME, Josephson CD, Konkle BA, Woodson RD, Ortel TL, Hillyer CD, Skerrett DL, McCrae KR, Sloan SR, Uhl L, George JN, Aquino VM, Manno CS, McFarland JG, Hess JR, Leissinger C, Granger S. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362(7):600–613. doi: 10.1056/NEJMoa0904084. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

