Development of new malaria diagnostics: matching performance and need
- PMID: 27515426
- PMCID: PMC4981959
- DOI: 10.1186/s12936-016-1454-8
Development of new malaria diagnostics: matching performance and need
Abstract
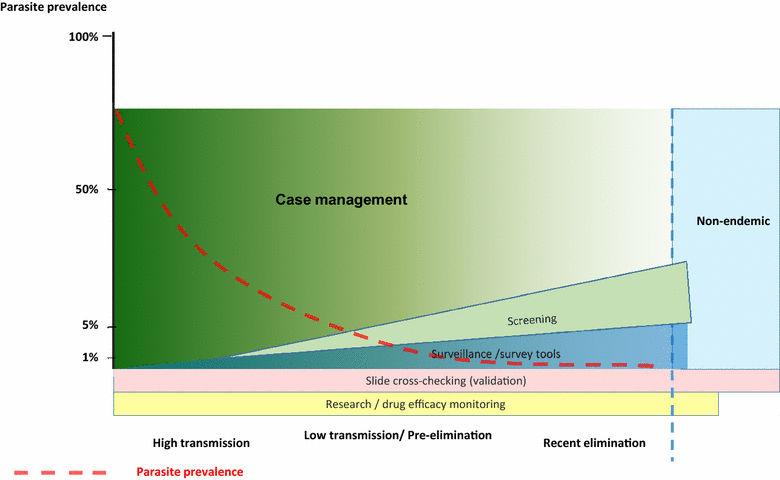
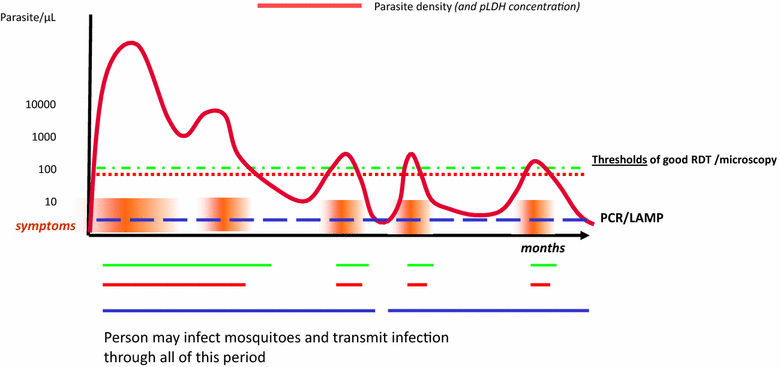
Despite advances in diagnostic technology, significant gaps remain in access to malaria diagnosis. Accurate diagnosis and misdiagnosis leads to unnecessary waste of resources, poor disease management, and contributes to a cycle of poverty in low-resourced communities. Despite much effort and investment, few new technologies have reached the field in the last 30 years aside from lateral flow assays. This suggests that much diagnostic development effort has been misdirected, and/or that there are fundamental blocks to introduction of new technologies. Malaria diagnosis is a difficult market; resources are broadly donor-dependent, health systems in endemic countries are frequently weak, and the epidemiology of malaria and priorities of malaria programmes and donors are evolving. Success in diagnostic development will require a good understanding of programme gaps, and the sustainability of markets to address them. Targeting assay development to such clearly defined market requirements will improve the outcomes of product development funding. Six market segments are identified: (1) case management in low-resourced countries, (2) parasite screening for low density infections in elimination programmes, (3) surveillance for evidence of continued transmission, (4) clinical research and therapeutic efficacy monitoring, (5) cross-checking for microscopy quality control, and (6) returned traveller markets distinguished primarily by resource availability. While each of these markets is potentially compelling from a public health standpoint, size and scale are highly variable and continue to evolve. Consequently, return on investment in research and development may be limited, highlighting the need for potentially significant donor involvement or the introduction of novel business models to overcome prohibitive economics. Given the rather specific applications, a well-defined set of stakeholders will need to be on board for the successful introduction and scaling of any new technology to these markets.
Keywords: Malaria; Malaria diagnostics market; Rapid diagnostic testing; Target product profiles.
Figures
Similar articles
-
Control of human parasitic diseases: Context and overview.Adv Parasitol. 2006;61:1-45. doi: 10.1016/S0065-308X(05)61001-9. Adv Parasitol. 2006. PMID: 16735161 Review.
-
Availability and price of malaria rapid diagnostic tests in the public and private health sectors in 2011: results from 10 nationally representative cross-sectional retail surveys.Trop Med Int Health. 2015 Jun;20(6):744-56. doi: 10.1111/tmi.12491. Epub 2015 Mar 22. Trop Med Int Health. 2015. PMID: 25728761
-
Acceptability of malaria rapid diagnostic tests administered by village health workers in Pangani District, North eastern Tanzania.Malar J. 2016 Aug 27;15(1):439. doi: 10.1186/s12936-016-1495-z. Malar J. 2016. PMID: 27567531 Free PMC article.
-
Malaria and the 'last' parasite: how can technology help?Malar J. 2018 Jul 11;17(1):260. doi: 10.1186/s12936-018-2408-0. Malar J. 2018. PMID: 29996831 Free PMC article. Review.
-
Malaria rapid diagnostic tests in travel medicine.Clin Microbiol Infect. 2013 May;19(5):408-15. doi: 10.1111/1469-0691.12152. Epub 2013 Feb 1. Clin Microbiol Infect. 2013. PMID: 23373854 Review.
Cited by
-
Performance of a sensitive haemozoin-based malaria diagnostic test validated for vivax malaria diagnosis in Brazilian Amazon.Malar J. 2021 Mar 12;20(1):146. doi: 10.1186/s12936-021-03688-0. Malar J. 2021. PMID: 33712019 Free PMC article. Clinical Trial.
-
Evaluation of the colorimetric malachite green loop-mediated isothermal amplification (MG-LAMP) assay for the detection of malaria species at two different health facilities in a malaria endemic area of western Kenya.Malar J. 2020 Sep 9;19(1):329. doi: 10.1186/s12936-020-03397-0. Malar J. 2020. PMID: 32907582 Free PMC article.
-
Recombinase Polymerase Amplification and Lateral Flow Assay for Ultrasensitive Detection of Low-Density Plasmodium falciparum Infection from Controlled Human Malaria Infection Studies and Naturally Acquired Infections.J Clin Microbiol. 2020 Apr 23;58(5):e01879-19. doi: 10.1128/JCM.01879-19. Print 2020 Apr 23. J Clin Microbiol. 2020. PMID: 32102854 Free PMC article.
-
International survey to identify diagnostic needs to support malaria elimination: guiding the development of combination highly sensitive rapid diagnostic tests.Malar J. 2017 Sep 22;16(1):385. doi: 10.1186/s12936-017-2037-z. Malar J. 2017. PMID: 28938906 Free PMC article.
-
The Diagnostic Performance of a Sysmex XN-31 Automated Malaria Analyzer vs. Expert Microscopy.Int J Lab Hematol. 2025 Aug;47(4):613-621. doi: 10.1111/ijlh.14456. Epub 2025 Mar 5. Int J Lab Hematol. 2025. PMID: 40042316 Free PMC article.
References
-
- WHO. World malaria report 2014. Geneva: World Health Organization; 2014. http://www.who.int/entity/malaria/publications/world_malaria_report_2014.... Accessed 2016.
-
- WHO. World malaria report 2015. Geneva: World Health Organization; 2015. http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf. Accessed 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

