Improving comfort in people with dementia and pneumonia: a cluster randomized trial
- PMID: 27515720
- PMCID: PMC4981997
- DOI: 10.1186/s12916-016-0663-x
Improving comfort in people with dementia and pneumonia: a cluster randomized trial
Abstract
Background: Pneumonia in people with dementia has been associated with severe discomfort. We sought to assess the effectiveness of a practice guideline for optimal symptom relief for nursing home residents with dementia and pneumonia.
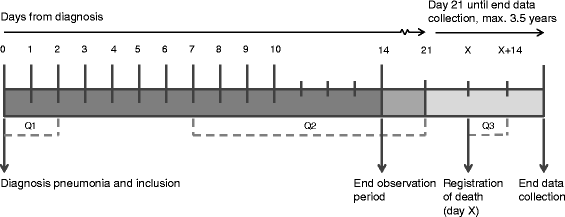
Methods: A single-blind, multicenter, cluster randomized controlled trial was conducted in 32 Dutch nursing homes. Outcomes were assessed on the patient level. The main outcome measures were discomfort and symptoms: discomfort (DS-DAT: Discomfort Scale-Dementia of Alzheimer Type), (lack of) comfort (EOLD-CAD: End Of Life in Dementia-Comfort Assessment in Dying), pain (PAINAD: Pain Assessment in Advanced Dementia), and respiratory distress (RDOS: Respiratory Distress Observation Scale). Outcomes were scheduled daily from diagnosis until 10 days later and a final time between 13-15 days from diagnosis by trained observers who were blinded to the intervention and the residents' condition and treatment. In a pre-intervention phase, usual care was provided to all homes. In the intervention phase, matched clusters of homes were randomized to either the control (n = 16) or intervention condition (n = 16).
Results: Between 1 January 2012 and 1 May 2015, 464 episodes of pneumonia were included. Outcomes were obtained for 399 episodes in 367 residents. Longitudinal multilevel linear regression analyses were performed on log-transformed outcomes, so coefficients should be interpreted as a ratio, and a coefficient of 1 means no difference. The practice guideline in the intervention phase did not reduce the level of discomfort and symptoms: DS-DAT: 1.11 (95 % CI 0.93-1.31), EOLD-CAD: 1.01 (95 % CI 0.98-1.05), PAINAD: 1.04 (95 % CI 0.93-1.15), RDOS: 1.11 (95 % CI 0.90-1.24). However, in both the intervention and control groups, lack of comfort and respiratory distress gradually decreased during the entire 3.5 years of data collection, and were lower in the intervention phase compared to the pre-intervention phase: DS-DAT: 0.93 (95 % CI 0.85-1.01), EOLD-CAD: 0.98 (95 % CI 0.97-1.00), PAINAD: 0.96 (95 % CI 0.91-1.01), RDOS: 0.92 (95 % CI 0.87-0.98).
Conclusions: When compared to usual care, the practice guideline for optimal symptom relief did not relieve discomfort and symptoms in nursing home residents with dementia and pneumonia. However, discomfort and symptoms decreased gradually throughout the data collection in both the intervention homes and the control homes. An intervention that focuses on creating awareness may be more effective than a physician practice guideline.
Trial registration: The Netherlands National Trial Register (ID number NTR5071 . Registered 10 March 2015).
Keywords: Dementia; Discomfort; Nursing homes; Pneumonia; Practice guideline; Trial.
Figures



References
-
- van der Maaden T, van der Steen JT, de Vet HC, Achterberg WP, Boersma F, Schols JM, et al. Development of a practice guideline for optimal symptom relief for patients with pneumonia and dementia in nursing homes using a Delphi study. Int J Geriatr Psychiatry. 2015;30(5):487–96. doi: 10.1002/gps.4167. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

