Elimination of microglia improves cognitive function following cranial irradiation
- PMID: 27516055
- PMCID: PMC4981848
- DOI: 10.1038/srep31545
Elimination of microglia improves cognitive function following cranial irradiation
Abstract
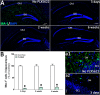
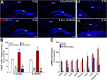
Cranial irradiation for the treatment of brain cancer elicits progressive and severe cognitive dysfunction that is associated with significant neuropathology. Radiation injury in the CNS has been linked to persistent microglial activation, and we find upregulation of pro-inflammatory genes even 6 weeks after irradiation. We hypothesize that depletion of microglia in the irradiated brain would have a neuroprotective effect. Adult mice received acute head only irradiation (9 Gy) and were administered a dietary inhibitor (PLX5622) of colony stimulating factor-1 receptor (CSF1R) to deplete microglia post-irradiation. Cohorts of mice maintained on a normal and PLX5662 diet were analyzed for cognitive changes using a battery of behavioral tasks 4-6 weeks later. PLX5622 treatment caused a rapid and near complete elimination of microglia in the brain within 3 days of treatment. Irradiation of animals given a normal diet caused characteristic behavioral deficits designed to test medial pre-frontal cortex (mPFC) and hippocampal learning and memory and caused increased microglial activation. Animals receiving the PLX5622 diet exhibited no radiation-induced cognitive deficits, and exhibited near complete loss of IBA-1 and CD68 positive microglia in the mPFC and hippocampus. Our data demonstrate that elimination of microglia through CSF1R inhibition can ameliorate radiation-induced cognitive deficits in mice.
Conflict of interest statement
B.L.W. is an employee of Plexxikon Inc. The remaining authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

