Reprogramming towards anabolism impedes degeneration in a preclinical model of retinitis pigmentosa
- PMID: 27516389
- PMCID: PMC5291198
- DOI: 10.1093/hmg/ddw256
Reprogramming towards anabolism impedes degeneration in a preclinical model of retinitis pigmentosa
Abstract
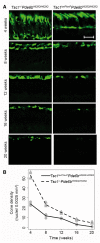
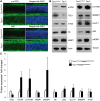
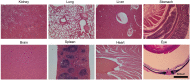
Retinitis pigmentosa (RP) is an incurable neurodegenerative condition featuring photoreceptor death that leads to blindness. Currently, there is no approved therapeutic for photoreceptor degenerative conditions like RP and atrophic age-related macular degeneration (AMD). Although there are promising results in human gene therapy, RP is a genetically diverse disorder, such that gene-specific therapies would be practical in a small fraction of patients with RP. Here, we explore a non-gene-specific strategy that entails reprogramming photoreceptors towards anabolism by upregulating the mechanistic target of rapamycin (mTOR) pathway. We conditionally ablated the tuberous sclerosis complex 1 (Tsc1) gene, an mTOR inhibitor, in the rods of the Pde6bH620Q/H620Q preclinical RP mouse model and observed, functionally and morphologically, an improvement in the survival of rods and cones at early and late disease stages. These results elucidate the ability of reprogramming the metabolome to slow photoreceptor degeneration. This strategy may also be applicable to a wider range of neurodegenerative diseases, as enhancement of nutrient uptake is not gene-specific and is implicated in multiple pathologies. Enhancing anabolism promoted neuronal survival and function and could potentially benefit a number of photoreceptor and other degenerative conditions.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





References
-
- Hartong D.T., Berson E.L., Dryja T.P. (2006) Retinitis pigmentosa. Lancet, 368, 1795–1809. - PubMed
-
- Fahim A.T., Daiger S.P., Weleber R.G. (1993) Pagon R.A., Adam M.P., Ardinger H.H., Wallace S.E., Amemiya A., Bean L.J.H., Bird T.D., Fong C.T., Mefford H.C., Smith R.J.H., Stephens K. (eds.), In GeneReviews(R), Seattle (WA: ), in press.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

