Exocyst SEC3 and Phosphoinositides Define Sites of Exocytosis in Pollen Tube Initiation and Growth
- PMID: 27516531
- PMCID: PMC5047084
- DOI: 10.1104/pp.16.00690
Exocyst SEC3 and Phosphoinositides Define Sites of Exocytosis in Pollen Tube Initiation and Growth
Abstract
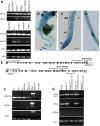
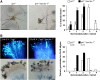
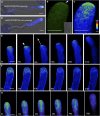
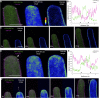
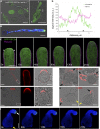
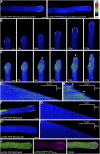
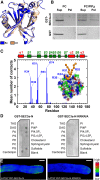
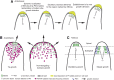
Polarized exocytosis is critical for pollen tube growth, but its localization and function are still under debate. The exocyst vesicle-tethering complex functions in polarized exocytosis. Here, we show that a sec3a exocyst subunit null mutant cannot be transmitted through the male gametophyte due to a defect in pollen tube growth. The green fluorescent protein (GFP)-SEC3a fusion protein is functional and accumulates at or proximal to the pollen tube tip plasma membrane. Partial complementation of sec3a resulted in the development of pollen with multiple tips, indicating that SEC3 is required to determine the site of pollen germination pore formation. Time-lapse imaging demonstrated that SEC3a and SEC8 were highly dynamic and that SEC3a localization on the apical plasma membrane predicts the direction of growth. At the tip, polar SEC3a domains coincided with cell wall deposition. Labeling of GFP-SEC3a-expressing pollen with the endocytic marker FM4-64 revealed the presence of subdomains on the apical membrane characterized by extensive exocytosis. In steady-state growing tobacco (Nicotiana tabacum) pollen tubes, SEC3a displayed amino-terminal Pleckstrin homology-like domain (SEC3a-N)-dependent subapical membrane localization. In agreement, SEC3a-N interacted with phosphoinositides in vitro and colocalized with a phosphatidylinositol 4,5-bisphosphate (PIP2) marker in pollen tubes. Correspondingly, molecular dynamics simulations indicated that SEC3a-N associates with the membrane by interacting with PIP2 However, the interaction with PIP2 is not required for polar localization and the function of SEC3a in Arabidopsis (Arabidopsis thaliana). Taken together, our findings indicate that SEC3a is a critical determinant of polar exocytosis during tip growth and suggest differential regulation of the exocytotic machinery depending on pollen tube growth modes.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Boavida LC, McCormick S (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52: 570–582 - PubMed
-
- Chebli Y, Geitmann A (2007) Mechanical principles governing pollen tube growth. Funct Plant Sci Biotechnol 1: 232–245
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

