Introduction and Utilization of High Priced HCV Medicines across Europe; Implications for the Future
- PMID: 27516740
- PMCID: PMC4964878
- DOI: 10.3389/fphar.2016.00197
Introduction and Utilization of High Priced HCV Medicines across Europe; Implications for the Future
Abstract
Background: Infection with the Hepatitis C Virus (HCV) is a widespread transmittable disease with a diagnosed prevalence of 2.0%. Fortunately, it is now curable in most patients. Sales of medicines to treat HCV infection grew 2.7% per year between 2004 and 2011, enhanced by the launch of the protease inhibitors (PIs) boceprevir (BCV) and telaprevir (TVR) in addition to ribavirin and pegylated interferon (pegIFN). Costs will continue to rise with new treatments including sofosbuvir, which now include interferon free regimens.
Objective: Assess the uptake of BCV and TVR across Europe from a health authority perspective to offer future guidance on dealing with new high cost medicines.
Methods: Cross-sectional descriptive study of medicines to treat HCV (pegIFN, ribavirin, BCV and TVR) among European countries from 2008 to 2013. Utilization measured in defined daily doses (DDDs)/1000 patients/quarter (DIQs) and expenditure in Euros/DDD. Health authority activities to influence treatments categorized using the 4E methodology (Education, Engineering, Economics and Enforcement).
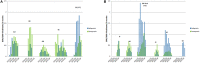
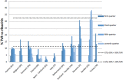
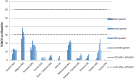
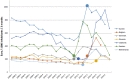
Results: Similar uptake of BCV and TVR among European countries and regions, ranging from 0.5 DIQ in Denmark, Netherlands and Slovenia to 1.5 DIQ in Tayside and Catalonia in 2013. However, different utilization of the new PIs vs. ribavirin indicates differences in dual vs. triple therapy, which is down to factors including physician preference and genotypes. Reimbursed prices for BCV and TVR were comparable across countries.
Conclusion: There was reasonable consistency in the utilization of BCV and TVR among European countries in comparison with other high priced medicines. This may reflect the social demand to limit the transmission of HCV. However, the situation is changing with new curative medicines for HCV genotype 1 (GT1) with potentially an appreciable budget impact. These concerns have resulted in different prices across countries, with their impact on budgets and patient outcomes monitored in the future to provide additional guidance.
Keywords: Hepatitis C; boceprevir; cross national drug utilization study; demand-side measures; introduction new medicines; sofosbuvir; telaprevir.
Figures
Similar articles
-
Cost-effectiveness of boceprevir or telaprevir for previously treated patients with genotype 1 chronic hepatitis C.J Hepatol. 2013 Oct;59(4):658-66. doi: 10.1016/j.jhep.2013.05.019. Epub 2013 May 23. J Hepatol. 2013. PMID: 23707373
-
A case of successful hepatitis C virus eradication by 24 weeks of telaprevir-based triple therapy for a hemophilia patient with hepatitis C virus/human immunodeficiency virus co-infection who previously failed pegylated interferon-α and ribavirin therapy.J Infect Chemother. 2014 May;20(5):320-4. doi: 10.1016/j.jiac.2013.11.006. Epub 2014 Jan 27. J Infect Chemother. 2014. PMID: 24477330
-
New agents for the treatment of hepatitis C in patients co-infected with HIV.Ther Adv Infect Dis. 2013 Apr;1(2):71-80. doi: 10.1177/2049936113479591. Ther Adv Infect Dis. 2013. PMID: 25165545 Free PMC article. Review.
-
Triple therapy with boceprevir or telaprevir in a European cohort of cirrhotic HIV/HCV genotype 1-coinfected patients.Liver Int. 2015 Sep;35(9):2090-9. doi: 10.1111/liv.12799. Epub 2015 Feb 23. Liver Int. 2015. PMID: 25650873
-
Hepatitis C genotype 4: The past, present, and future.World J Hepatol. 2015 Dec 8;7(28):2792-810. doi: 10.4254/wjh.v7.i28.2792. World J Hepatol. 2015. PMID: 26668691 Free PMC article. Review.
Cited by
-
Health utilities using SF-6D scores in Japanese patients with chronic hepatitis C treated with sofosbuvir-based regimens in clinical trials.Health Qual Life Outcomes. 2017 Jan 31;15(1):25. doi: 10.1186/s12955-017-0598-8. Health Qual Life Outcomes. 2017. PMID: 28143559 Free PMC article.
-
Conceptualizing Care Continua: Lessons from HIV, Hepatitis C Virus, Tuberculosis and Implications for the Development of Improved Care and Prevention Continua.Front Public Health. 2017 Jan 10;4:296. doi: 10.3389/fpubh.2016.00296. eCollection 2016. Front Public Health. 2017. PMID: 28119910 Free PMC article. Review.
-
Projecting Pharmaceutical Expenditure in EU5 to 2021: Adjusting for the Impact of Discounts and Rebates.Appl Health Econ Health Policy. 2018 Dec;16(6):803-817. doi: 10.1007/s40258-018-0419-1. Appl Health Econ Health Policy. 2018. PMID: 30088251 Free PMC article.
-
Payers' Views of the Changes Arising through the Possible Adoption of Adaptive Pathways.Front Pharmacol. 2016 Sep 28;7:305. doi: 10.3389/fphar.2016.00305. eCollection 2016. Front Pharmacol. 2016. PMID: 27733828 Free PMC article.
-
Barriers for Access to New Medicines: Searching for the Balance Between Rising Costs and Limited Budgets.Front Public Health. 2018 Dec 5;6:328. doi: 10.3389/fpubh.2018.00328. eCollection 2018. Front Public Health. 2018. PMID: 30568938 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

