Mesenchymal Stromal Cells from Osteoarthritic Synovium Are a Distinct Population Compared to Their Bone-Marrow Counterparts regarding Surface Marker Distribution and Immunomodulation of Allogeneic CD4+ T-Cell Cultures
- PMID: 27516777
- PMCID: PMC4969547
- DOI: 10.1155/2016/6579463
Mesenchymal Stromal Cells from Osteoarthritic Synovium Are a Distinct Population Compared to Their Bone-Marrow Counterparts regarding Surface Marker Distribution and Immunomodulation of Allogeneic CD4+ T-Cell Cultures
Abstract
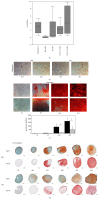
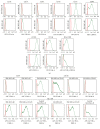
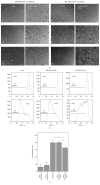
Introduction. The participation of an inflammatory joint milieu has been described in osteoarthritis (OA) pathogenesis. Mesenchymal stromal cells (MSCs) play an important role in modulating inflammatory processes. Based on previous studies in an allogeneic T-cell coculture model, we aimed at further determining the role of synovial MSCs in OA pathogenesis. Methods. Bone-marrow (BM) and synovial membrane (SM) MSCs from hip joints of late stage OA patients and CD4+ T-cells from healthy donors were analysed regarding surface marker expression before and after coculture. Proliferation upon CD3/CD28 stimulation and cytokine analyses were compared between MSCs. Results. SM-MSCs differed from BM-MSCs in several surface markers and their osteogenic differentiation potential. Cocultures of both MSCs with CD4+ T-cells resulted in recruitment of CD45RA+ FoxP3+ regulatory T-cells. Upon stimulation, only SM-MSCs suppressed CD4+ T-cell proliferation, while both SM-MSCs and BM-MSCs modified cytokine profiles through suppressing IL-2 and TNF-α as well as increasing IL-6 secretion. Conclusions. Synovial MSCs from OA joints are a unique fraction that can be distinguished from their bone-marrow derived counterparts. Their unique ability to suppress CD3/CD28 induced CD4+ T-cell proliferation makes them a potential target for future therapeutic approaches.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

