Successful alectinib treatment after crizotinib-induced interstitial lung disease
- PMID: 27516885
- PMCID: PMC4968663
- DOI: 10.1002/rcr2.156
Successful alectinib treatment after crizotinib-induced interstitial lung disease
Abstract
A 70-year-old woman with lung adenocarcinoma, harbouring anaplastic lymphoma kinase gene rearrangement, was treated with crizotinib as third-line chemotherapy. After 2 months, crizotinib was discontinued because of the development of crizotinib-induced interstitial lung disease (ILD). Steroid treatment was then introduced and tapered off. Following complete resolution of the interstitial shadow, cytotoxic chemotherapy was initiated, and continued for over 2 years, until new intrapulmonary lesions developed. Although there was a risk of drug-induced interstitial pneumonia, alectinib was initiated as the fifth-line therapy, without steroid supplementation, as there was no alternative treatment. No recurrence of ILD was noted at 10 months. To our knowledge, this is the first report of successful alectinib treatment after the development of crizotinib-induced ILD without the use of prednisolone.
Keywords: anaplastic lymphoma kinase‐tyrosine kinase inhibitor; interstitial lung disease.
Figures
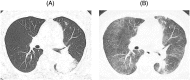
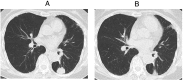
References
-
- Crequit P, Wislez M, Fleury Feith J, et al. 2015. Crizotinib associated with ground‐glass opacity predominant pattern interstitial lung disease: a retrospective observational cohort study with a systematic literature review. J. Thoracic Oncol. 10:1148–1155. - PubMed
-
- Harada C, Kawaguchi T, Ogata‐Suetsugu S, et al. 2011. EGFR tyrosine kinase inhibition worsens acute lung injury in mice with repairing airway epithelium. Am. J. Respir. Crit. Care Med. 183:743–751. - PubMed
-
- Kitajima H, Takahashi H, Harada K, et al. 2006. Gefitinib‐induced interstitial lung disease showing improvement after cessation: disassociation of serum markers. Respirology 11:217–220. - PubMed
-
- Yanagisawa S, Inoue A, Koarai A, et al. 2013. Successful crizotinib retreatment after crizotinib‐induced interstitial lung disease. J. Thoracic Oncol. 8:e73–e74. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

