The Habituation/Cross-Habituation Test Revisited: Guidance from Sniffing and Video Tracking
- PMID: 27516910
- PMCID: PMC4969543
- DOI: 10.1155/2016/9131284
The Habituation/Cross-Habituation Test Revisited: Guidance from Sniffing and Video Tracking
Abstract
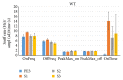
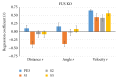
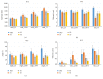
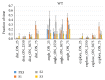
The habituation/cross-habituation test (HaXha) is a spontaneous odor discrimination task that has been used for many decades to evaluate olfactory function in animals. Animals are presented repeatedly with the same odorant after which a new odorant is introduced. The time the animal explores the odor object is measured. An animal is considered to cross-habituate during the novel stimulus trial when the exploration time is higher than the prior trial and indicates the degree of olfactory patency. On the other hand, habituation across the repeated trials involves decreased exploration time and is related to memory patency, especially at long intervals. Classically exploration is timed using a stopwatch when the animal is within 2 cm of the object and aimed toward it. These criteria are intuitive, but it is unclear how they relate to olfactory exploration, that is, sniffing. We used video tracking combined with plethysmography to improve accuracy, avoid observer bias, and propose more robust criteria for exploratory scoring when sniff measures are not available. We also demonstrate that sniff rate combined with proximity is the most direct measure of odorant exploration and provide a robust and sensitive criterion.
Figures








Similar articles
-
Sniffing behavior of mice during performance in odor-guided tasks.Chem Senses. 2008 Sep;33(7):581-96. doi: 10.1093/chemse/bjn029. Epub 2008 Jun 5. Chem Senses. 2008. PMID: 18534995 Free PMC article.
-
Testing for odor discrimination and habituation in mice.J Vis Exp. 2015 May 5;(99):e52615. doi: 10.3791/52615. J Vis Exp. 2015. PMID: 25992586 Free PMC article.
-
Habituation of stimulus-elicited investigation in gerbils after olfactory bulbectomy.Physiol Behav. 1979 Aug;23(2):309-15. doi: 10.1016/0031-9384(79)90372-x. Physiol Behav. 1979. PMID: 504421 No abstract available.
-
Olfactory habituation in Drosophila-odor encoding and its plasticity in the antennal lobe.Prog Brain Res. 2014;208:3-38. doi: 10.1016/B978-0-444-63350-7.00001-2. Prog Brain Res. 2014. PMID: 24767477 Review.
-
The sniff as a unit of olfactory processing.Chem Senses. 2006 Feb;31(2):167-79. doi: 10.1093/chemse/bjj016. Epub 2005 Dec 8. Chem Senses. 2006. PMID: 16339265 Review.
Cited by
-
Fus1 KO Mouse As a Model of Oxidative Stress-Mediated Sporadic Alzheimer's Disease: Circadian Disruption and Long-Term Spatial and Olfactory Memory Impairments.Front Aging Neurosci. 2016 Nov 15;8:268. doi: 10.3389/fnagi.2016.00268. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27895577 Free PMC article.
-
The functional relevance of olfactory marker protein in the vertebrate olfactory system: a never-ending story.Cell Tissue Res. 2021 Jan;383(1):409-427. doi: 10.1007/s00441-020-03349-9. Epub 2021 Jan 15. Cell Tissue Res. 2021. PMID: 33447880 Free PMC article. Review.
-
A Comparison between Mouse, In Silico, and Robot Odor Plume Navigation Reveals Advantages of Mouse Odor Tracking.eNeuro. 2020 Feb 4;7(1):ENEURO.0212-19.2019. doi: 10.1523/ENEURO.0212-19.2019. Print 2020 Jan/Feb. eNeuro. 2020. PMID: 31924732 Free PMC article.
-
Neurogenesis dynamics in the olfactory bulb: deciphering circuitry organization, function, and adaptive plasticity.Neural Regen Res. 2025 Jun 1;20(6):1565-1581. doi: 10.4103/NRR.NRR-D-24-00312. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 38934393 Free PMC article.
-
Wireless monitoring of respiration with EEG reveals relationships between respiration, behavior, and brain activity in freely moving mice.J Neurophysiol. 2024 Jul 1;132(1):290-307. doi: 10.1152/jn.00330.2023. Epub 2024 May 29. J Neurophysiol. 2024. PMID: 38810259 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

