A suggested vital function for eIF-5A and dhs genes during murine malaria blood-stage infection
- PMID: 27516964
- PMCID: PMC4971841
- DOI: 10.1002/2211-5463.12093
A suggested vital function for eIF-5A and dhs genes during murine malaria blood-stage infection
Abstract
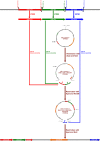
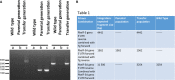
The biological function of the post-translational modification hypusine in the eukaryotic initiation factor 5A (EIF-5A) in eukaryotes is still not understood. Hypusine is formed by two sequential enzymatic steps at a specific lysine residue in the precursor protein EIF-5A. One important biological function of EIF-5A which was recently identified is the translation of polyproline-rich mRNA, suggesting its biological relevance in a variety of biological processes. Hypusinated eIF-5A controls the proliferation of cancer cells and inflammatory processes in malaria. It was shown that pharmacological inhibition of the enzymes involved in this pathway, deoxyhypusine synthase (DHS) and the deoxyhypusine hydroxylase (DOHH), arrested the growth of malaria parasites. Down-regulation of both the malarial eIF-5A and dhs genes by in vitro and in vivo silencing led to decreased transcript levels and protein expression and, in turn, to low parasitemia, confirming a critical role of hypusination in eIF-5A for proliferation in Plasmodium. To further investigate whether eIF-5A and the activating enzyme DHS are essential for Plasmodium erythrocytic stages, targeted gene disruption was performed in the rodent malaria parasite Plasmodium berghei. Full disruption of both genes was not successful; instead parasites harboring the episome for eIF-5A and dhs genes were obtained, suggesting that these genes may perform vital functions during the pathogenic blood cell stage. Next, a knock-in strategy was pursued for both endogenous genes eIF-5A and dhs from P. berghei. The latter resulted in viable recombinant parasites, strengthening the observation that they might be essential for proliferation during asexual development of the malaria parasite.
Keywords: Plasmodium; hypusine; malaria; reverse genetics.
Figures





Similar articles
-
In vitro and in vivo silencing of plasmodial dhs and eIf-5a genes in a putative, non-canonical RNAi-related pathway.BMC Microbiol. 2012 Jun 13;12:107. doi: 10.1186/1471-2180-12-107. BMC Microbiol. 2012. PMID: 22694849 Free PMC article.
-
Modification of eukaryotic initiation factor 5A from Plasmodium vivax by a truncated deoxyhypusine synthase from Plasmodium falciparum: An enzyme with dual enzymatic properties.Bioorg Med Chem. 2007 Sep 15;15(18):6200-7. doi: 10.1016/j.bmc.2007.06.026. Epub 2007 Jun 14. Bioorg Med Chem. 2007. PMID: 17591443
-
Hypusine: its post-translational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation.Biofactors. 1993 May;4(2):95-104. Biofactors. 1993. PMID: 8347280 Review.
-
Piperidones with activity against Plasmodium falciparum.Parasitol Res. 2006 Aug;99(3):281-6. doi: 10.1007/s00436-006-0173-4. Epub 2006 Mar 21. Parasitol Res. 2006. PMID: 16550432
-
Chemical profiling of deoxyhypusine hydroxylase inhibitors for antimalarial therapy.Amino Acids. 2013 Nov;45(5):1047-53. doi: 10.1007/s00726-013-1575-0. Epub 2013 Aug 14. Amino Acids. 2013. PMID: 23943044 Review.
Cited by
-
Validation of Plasmodium falciparum deoxyhypusine synthase as an antimalarial target.PeerJ. 2019 Apr 17;7:e6713. doi: 10.7717/peerj.6713. eCollection 2019. PeerJ. 2019. PMID: 31024761 Free PMC article.
-
An experimental target-based platform in yeast for screening Plasmodium vivax deoxyhypusine synthase inhibitors.PLoS Negl Trop Dis. 2024 Dec 2;18(12):e0012690. doi: 10.1371/journal.pntd.0012690. eCollection 2024 Dec. PLoS Negl Trop Dis. 2024. PMID: 39621767 Free PMC article.
-
The Role of Spermidine and Its Key Metabolites in Important, Pathogenic Human Viruses and in Parasitic Infections Caused by Plasmodium falciparum and Trypanosoma brucei.Biomolecules. 2023 May 9;13(5):803. doi: 10.3390/biom13050803. Biomolecules. 2023. PMID: 37238673 Free PMC article. Review.
-
Investigation of an Allosteric Deoxyhypusine Synthase Inhibitor in P. falciparum.Molecules. 2022 Apr 11;27(8):2463. doi: 10.3390/molecules27082463. Molecules. 2022. PMID: 35458660 Free PMC article.
References
-
- WHO malaria report. www.who.int/entity/malaria/publications/world-malaria-report-2015/report/en.
-
- Kronenberger T, Lindner J, Meissner KA, Zimbres FM, Coronado MA, Sauer FM, Schettert I and Wrenger C (2014) Vitamin B6‐dependent enzymes in the human malaria parasite Plasmodium falciparum: a druggable target? Biomed Res Int 2014, 108516. doi: 10.1155/2014/108516. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

