APP Overexpression Causes Aβ-Independent Neuronal Death through Intrinsic Apoptosis Pathway
- PMID: 27517085
- PMCID: PMC4967816
- DOI: 10.1523/ENEURO.0150-16.2016
APP Overexpression Causes Aβ-Independent Neuronal Death through Intrinsic Apoptosis Pathway
Abstract
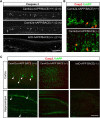
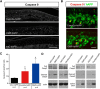
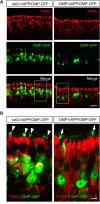
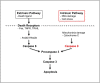
Accumulation of amyloid-β (Aβ) peptide in the brain is a central hallmark of Alzheimer's disease (AD) and is thought to be the cause of the observed neurodegeneration. Many animal models have been generated that overproduce Aβ yet do not exhibit clear neuronal loss, questioning this Aβ hypothesis. We previously developed an in vivo mouse model that expresses a humanized amyloid precursor protein (hAPP) in olfactory sensory neurons (OSNs) showing robust apoptosis and olfactory dysfunction by 3 weeks of age, which is consistent with early OSN loss and smell deficits, as observed in AD patients. Here we show, by deleting the β-site APP cleaving enzyme 1 (BACE1) in two distinct transgenic mouse models, that hAPP-induced apoptosis of OSNs is Aβ independent and remains cell autonomous. In addition, we reveal that the intrinsic apoptosis pathway is responsible for hAPP-induced OSN death, as marked by mitochondrial damage and caspase-9 activation. Given that hAPP expression causes OSN apoptosis despite the absence of BACE1, we propose that Aβ is not the sole cause of hAPP-induced neurodegeneration and that the early loss of olfactory function in AD may be based on a cell-autonomous mechanism, which could mark an early phase of AD, prior to Aβ accumulation. Thus, the olfactory system could serve as an important new platform to study the development of AD, providing unique insight for both early diagnosis and intervention.
Keywords: amyloid precursor protein; apoptosis; neurodegeneration; olfactory.
Figures



References
-
- Bacon AW, Bondi MW, Salmon DP, Murphy C (1998) Very early changes in olfactory functioning due to Alzheimer's disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci 855:723–731. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
