Involvement of urokinase receptor in the cross-talk between human hematopoietic stem cells and bone marrow microenvironment
- PMID: 27517491
- PMCID: PMC5312379
- DOI: 10.18632/oncotarget.11115
Involvement of urokinase receptor in the cross-talk between human hematopoietic stem cells and bone marrow microenvironment
Abstract
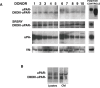
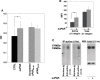
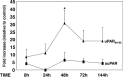
Hematopoietic stem cells (HSCs) reside in bone marrow (BM) and can be induced to mobilize into the circulation for transplantation. Homing and lodgement into BM of transplanted HSCs are the first critical steps in their engraftment and involve multiple interactions between HSCs and the BM microenvironment.uPAR is a three domain receptor (DIDIIDIII) which binds urokinase, vitronectin, integrins. uPAR can be cleaved and shed from the cell surface generating full-length and cleaved soluble forms (suPAR and DIIDIII-suPAR). DIIDIII-suPAR can bind fMLF receptors through the SRSRY sequence (residues 88-92).We previously reported the involvement of soluble uPAR in HSC mobilization. We now investigate its possible role in HSC homing and engraftment.We show similar levels of circulating full-length suPAR in healthy donors and in acute myeloid leukemia (AML) patients before and after the pre-transplant conditioning regimen. By contrast, levels of circulating DIIDIII-suPAR in AML patients are higher as compared to controls and significantly decrease after the conditioning.We found that suPAR and uPAR84-95, a uPAR-derived peptide which mimics active DIIDIII-suPAR, induce a significant increase in Long Term Culture (LTC)-Initiating Cells (ICs) and in the release of clonogenic progenitors from LTCs of CD34+ HSCs. Further, suPAR increases adhesion and survival of CD34+ KG1 AML cells, whereas uPAR84-95 increases their proliferation.Thus, circulating DIIDIII-suPAR, strongly increased in HSC mobilization, is indeed down-regulated by pre-transplant conditioning, probably to favour HSC homing. BM full-length suPAR and DIIDIII-suPAR may be involved in HSC lodgement within the BM by contributing to a suitable microenvironment.
Keywords: bone marrow microenvironment; hematopoietic stem cells; leukemia; uPAR; urokinase receptor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Garrett RW, Emerson SG. Bone and blood vessels: the hard and the soft of hematopoietic stem cell niches. Cell Stem Cell. 2009;4:503–6. - PubMed
-
- Battiwalla M, McCarthy PL. Filgrastim support in allogeneic HSCT for myeloid malignancies: a review of the role of G-CSF and the implications for current practice. Bone Marrow Transplant. 2009;43:351–6. - PubMed
-
- Selleri C, Montuori N, Ricci P, Visconte V, Carriero MV, Sidenius N, Serio B, Blasi F, Rotoli B, Rossi G, Ragno P. Involvement of the urokinase-type plasminogen activator receptor in hematopoietic stem cell mobilization. Blood. 2005;105:2198–205. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

