Flavonoids as CDK1 Inhibitors: Insights in Their Binding Orientations and Structure-Activity Relationship
- PMID: 27517610
- PMCID: PMC4982677
- DOI: 10.1371/journal.pone.0161111
Flavonoids as CDK1 Inhibitors: Insights in Their Binding Orientations and Structure-Activity Relationship
Abstract
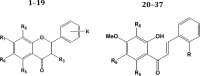
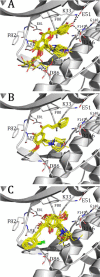
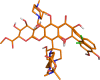
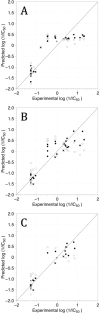
In the last years, the interactions of flavonoids with protein kinases (PKs) have been described by using crystallographic experiments. Interestingly, different orientations have been found for one flavonoid inside different PKs and different chemical substitutions lead to different orientations of the flavonoid scaffold inside one PK. Accordingly, orientation predictions of novel analogues could help to the design of flavonoids with high PK inhibitory activities. With this in mind, we studied the binding modes of 37 flavonoids (flavones and chalcones) inside the cyclin-dependent PK CDK1 using docking experiments. We found that the compounds under study adopted two different orientations into the active site of CDK1 (orientations I and II in the manuscript). In addition, quantitative structure-activity relationship (QSAR) models using CoMFA and CoMSIA methodologies were constructed to explain the trend of the CDK1 inhibitory activities for the studied flavonoids. Template-based and docking-based alignments were used. Models developed starting from docking-based alignment were applied for describing the whole dataset and compounds with orientation I. Adequate R2 and Q2 values were obtained by each method; interestingly, only hydrophobic and hydrogen bond donor fields describe the differential potency of the flavonoids as CDK1 inhibitors for both defined alignments and subsets. Our current application of docking and QSAR together reveals important elements to be drawn for the design of novel flavonoids with increased PK inhibitory activities.
Conflict of interest statement
Figures
Similar articles
-
Homology modeling, molecular dynamic simulation and docking studies of cyclin dependent kinase 1.J Mol Model. 2011 Feb;17(2):219-26. doi: 10.1007/s00894-010-0710-z. Epub 2010 Apr 26. J Mol Model. 2011. PMID: 20419498
-
3D-QSAR and docking studies of flavonoids as potent Escherichia coli inhibitors.Sci Rep. 2016 Apr 6;6:23634. doi: 10.1038/srep23634. Sci Rep. 2016. PMID: 27049530 Free PMC article.
-
3D-QSAR (CoMFA and CoMSIA) and pharmacophore (GALAHAD) studies on the differential inhibition of aldose reductase by flavonoid compounds.J Mol Graph Model. 2010 Nov;29(3):363-71. doi: 10.1016/j.jmgm.2010.08.005. Epub 2010 Sep 21. J Mol Graph Model. 2010. PMID: 20863730
-
SAR, QSAR and docking of anticancer flavonoids and variants: a review.Curr Top Med Chem. 2012;12(24):2785-809. doi: 10.2174/1568026611212240007. Curr Top Med Chem. 2012. PMID: 23368103 Review.
-
GABA(A)-receptor ligands of flavonoid structure.Curr Top Med Chem. 2002 Aug;2(8):853-67. doi: 10.2174/1568026023393462. Curr Top Med Chem. 2002. PMID: 12171576 Review.
Cited by
-
Docking, Interaction Fingerprint, and Three-Dimensional Quantitative Structure-Activity Relationship (3D-QSAR) of Sigma1 Receptor Ligands, Analogs of the Neuroprotective Agent RC-33.Front Chem. 2019 Jul 11;7:496. doi: 10.3389/fchem.2019.00496. eCollection 2019. Front Chem. 2019. PMID: 31355187 Free PMC article.
-
Computational identification of potential inhibitors targeting cdk1 in colorectal cancer.Front Chem. 2023 Nov 30;11:1264808. doi: 10.3389/fchem.2023.1264808. eCollection 2023. Front Chem. 2023. PMID: 38099190 Free PMC article.
-
An unconventional gatekeeper mutation sensitizes inositol hexakisphosphate kinases to an allosteric inhibitor.Elife. 2023 Oct 16;12:RP88982. doi: 10.7554/eLife.88982. Elife. 2023. PMID: 37843983 Free PMC article.
-
From cell cycle control to cancer therapy: exploring the role of CDK1 and CDK2 in tumorigenesis.Med Oncol. 2025 Aug 9;42(9):422. doi: 10.1007/s12032-025-02973-1. Med Oncol. 2025. PMID: 40782258 Review.
-
Mur ligase F as a new target for the flavonoids quercitrin, myricetin, and (-)-epicatechin.J Comput Aided Mol Des. 2023 Dec;37(12):721-733. doi: 10.1007/s10822-023-00535-z. Epub 2023 Oct 5. J Comput Aided Mol Des. 2023. PMID: 37796382 Free PMC article.
References
-
- Hollman PC, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 1999;37: 937–942. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

