Inhibition of glucosylceramide synthase eliminates the oncogenic function of p53 R273H mutant in the epithelial-mesenchymal transition and induced pluripotency of colon cancer cells
- PMID: 27517620
- PMCID: PMC5312403
- DOI: 10.18632/oncotarget.11169
Inhibition of glucosylceramide synthase eliminates the oncogenic function of p53 R273H mutant in the epithelial-mesenchymal transition and induced pluripotency of colon cancer cells
Abstract
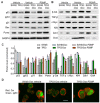
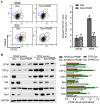
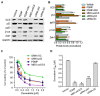
Missense mutation of tumor suppressor p53, which exhibits oncogenic gain-of-function (GOF), not only promotes tumor progression, but also diminishes therapeutic efficacies of cancer treatments. However, it remains unclear how a p53 missense mutant contributes to induced pluripotency of cancer stem cells (CSCs) in tumors exposed to chemotherapeutic agents. More importantly, it may be possible to abrogate the GOF by restoring wild-type p53 activity, thereby overcoming the deleterious effects resulting from heterotetramer formation, which often compromises the efficacies of current approaches being used to reactivate p53 function. Herewith, we report that p53 R273H missense mutant urges cancer cells to spawn CSCs. SW48/TP53 cells, which heterozygously carry the p53 R273H hot-spot mutant (R273H/+, introduced by a CRISPR/Casp9 system), were subchronically exposed to doxorubicin in cell culture and in tumor-bearing mice. We found that p53-R273H (TP53-Dox) cells were drug-resistant and exhibited epithelial-mesenchymal transition (EMT) and increased numbers of CSCs (CD44v6+/CD133+), which resulted in enhanced wound healing and tumor formation. Inhibition of glucosylceramide synthase with d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) sensitized p53-R273H cancer cells and tumor xenografts to doxorubicin treatments. Intriguingly, PDMP treatments restored wild-type p53 expression in heterozygous R273H mutant cells and in tumors, decreasing CSCs and sensitizing cells and tumors to treatments. This study demonstrated that p53-R273H promotes EMT and induced pluripotency of CSCs in cancer cells exposed to doxorubicin, mainly through Zeb1 and β-catenin transcription factors. Our results further indicate that restoration of p53 through inhibition of ceramide glycosylation might be an effective treatment approach for targeting cancers heterozygously harboring TP53 missense mutations.
Keywords: cancer stem cells; epithelial-mesenchymal transition; glucosylceramide synthase; missense mutation; tumor suppressor p53.
Conflict of interest statement
The authors do not have any conflict of interest.
Figures






Similar articles
-
An N6-methyladenosine at the transited codon 273 of p53 pre-mRNA promotes the expression of R273H mutant protein and drug resistance of cancer cells.Biochem Pharmacol. 2019 Feb;160:134-145. doi: 10.1016/j.bcp.2018.12.014. Epub 2018 Dec 19. Biochem Pharmacol. 2019. PMID: 30578766 Free PMC article.
-
P53-R273H mutation enhances colorectal cancer stemness through regulating specific lncRNAs.J Exp Clin Cancer Res. 2019 Aug 28;38(1):379. doi: 10.1186/s13046-019-1375-9. J Exp Clin Cancer Res. 2019. PMID: 31455383 Free PMC article.
-
Gb3-cSrc complex in glycosphingolipid-enriched microdomains contributes to the expression of p53 mutant protein and cancer drug resistance via β-catenin-activated RNA methylation.FASEB Bioadv. 2020 Sep 2;2(11):653-667. doi: 10.1096/fba.2020-00044. eCollection 2020 Nov. FASEB Bioadv. 2020. PMID: 33205006 Free PMC article.
-
Mutant p53 in colon cancer.J Mol Cell Biol. 2019 Apr 1;11(4):267-276. doi: 10.1093/jmcb/mjy075. J Mol Cell Biol. 2019. PMID: 30496442 Free PMC article. Review.
-
Emerging Non-Canonical Functions and Regulation by p53: p53 and Stemness.Int J Mol Sci. 2016 Nov 26;17(12):1982. doi: 10.3390/ijms17121982. Int J Mol Sci. 2016. PMID: 27898034 Free PMC article. Review.
Cited by
-
An N6-methyladenosine at the transited codon 273 of p53 pre-mRNA promotes the expression of R273H mutant protein and drug resistance of cancer cells.Biochem Pharmacol. 2019 Feb;160:134-145. doi: 10.1016/j.bcp.2018.12.014. Epub 2018 Dec 19. Biochem Pharmacol. 2019. PMID: 30578766 Free PMC article.
-
Targeting glucosylceramide synthase induces antiproliferative and proapoptotic effects in osimertinib-resistant NSCLC cell models.Sci Rep. 2024 Mar 18;14(1):6491. doi: 10.1038/s41598-024-57028-8. Sci Rep. 2024. PMID: 38499619 Free PMC article.
-
Lipid metabolic Reprogramming: Role in Melanoma Progression and Therapeutic Perspectives.Cancers (Basel). 2020 Oct 27;12(11):3147. doi: 10.3390/cancers12113147. Cancers (Basel). 2020. PMID: 33121001 Free PMC article. Review.
-
CRISPR/Cas9 for overcoming drug resistance in solid tumors.Daru. 2020 Jun;28(1):295-304. doi: 10.1007/s40199-019-00240-z. Epub 2019 Jan 21. Daru. 2020. PMID: 30666557 Free PMC article. Review.
-
Plakoglobin restores tumor suppressor activity of p53R175H mutant by sequestering the oncogenic potential of β-catenin.Cancer Sci. 2018 Jun;109(6):1876-1888. doi: 10.1111/cas.13612. Epub 2018 May 23. Cancer Sci. 2018. PMID: 29660231 Free PMC article.
References
-
- Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. - PubMed
-
- Leroy B, Anderson M, Soussi T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum Mutat. 2014;35:672–688. - PubMed
-
- Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15:2–8. - PubMed
-
- Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, Bigner SH, Davidson N, Baylin S, Devilee P, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989;342:705–708. - PubMed
-
- Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

