In Vitro Antileishmanial Activity of Sterols from Trametes versicolor (Bres. Rivarden)
- PMID: 27517895
- PMCID: PMC6273698
- DOI: 10.3390/molecules21081045
In Vitro Antileishmanial Activity of Sterols from Trametes versicolor (Bres. Rivarden)
Abstract
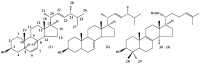
Two ergostanes, 5α,8α-epidioxy-22E-ergosta-6,22-dien-3β-ol (1) and 5α-ergost-7,22-dien-3β-ol (2), and a lanostane, 3β-hydroxylanostan-8,24-diene-21-oic acid (trametenolic acid) (3), were isolated from an n-hexane extract prepared from the fruiting body of Trametes versicolor (Bres. Rivarden). The activity of the isolated sterols was evaluated against promastigotes and amastigotes of Leishmania amazonensis Lainson and Shaw, 1972. The lanostane, compound (3), showed the best inhibitory response (IC50 promastigotes 2.9 ± 0.1 μM and IC50 amastigotes 1.6 ± 0.1 μM). This effect was 25-fold higher compared with its cytotoxic effect on peritoneal macrophages from BALB/c mice. Therefore, trametenolic acid could be regarded as a promising lead for the synthesis of compounds with antileishmanial activity.
Keywords: Trametes versicolor; antileishmanial activity; trametenolic acid B.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
C28 steroids from the fruiting bodies of Ganoderma resinaceum with potential anti-inflammatory activity.Phytochemistry. 2019 Dec;168:112109. doi: 10.1016/j.phytochem.2019.112109. Epub 2019 Sep 5. Phytochemistry. 2019. PMID: 31494344
-
Cytotoxic sterols from marine-derived fungus Pennicillium sp.Nat Prod Res. 2006 Apr;20(4):381-4. doi: 10.1080/14786410600661229. Nat Prod Res. 2006. PMID: 16644533
-
3β,5α,6β-Oxygenated sterols from the South China Sea gorgonian Muriceopsis flavida and their tumor cell growth inhibitory activity and apoptosis-inducing function.Steroids. 2013 Jan;78(1):108-14. doi: 10.1016/j.steroids.2012.10.003. Epub 2012 Nov 2. Steroids. 2013. PMID: 23123740
-
Morinda citrifolia Linn. fruit (Noni) juice induces an increase in NO production and death of Leishmania amazonensis amastigotes in peritoneal macrophages from BALB/c.Nitric Oxide. 2016 Aug 31;58:51-8. doi: 10.1016/j.niox.2016.06.004. Epub 2016 Jun 18. Nitric Oxide. 2016. PMID: 27328771 Review.
-
Ergostanes from the mushroom Trametes versicolor and their cancer cell inhibition: In vitro and in silico evaluation.Steroids. 2024 Dec;212:109511. doi: 10.1016/j.steroids.2024.109511. Epub 2024 Sep 19. Steroids. 2024. PMID: 39303896 Review.
Cited by
-
Composition of Triterpenoids in Inonotus obliquus and Their Anti-Proliferative Activity on Cancer Cell Lines.Molecules. 2020 Sep 6;25(18):4066. doi: 10.3390/molecules25184066. Molecules. 2020. PMID: 32899899 Free PMC article.
-
Bioactives from Mushroom: Health Attributes and Food Industry Applications.Materials (Basel). 2021 Dec 11;14(24):7640. doi: 10.3390/ma14247640. Materials (Basel). 2021. PMID: 34947237 Free PMC article. Review.
-
Submerged cultivation and phytochemical analysis of medicinal mushrooms (Trametes sp.).Front Fungal Biol. 2024 Jun 11;5:1414349. doi: 10.3389/ffunb.2024.1414349. eCollection 2024. Front Fungal Biol. 2024. PMID: 38919599 Free PMC article.
-
Ergosterol Peroxide from the Medicinal Mushroom Ganoderma lucidum Inhibits Differentiation and Lipid Accumulation of 3T3-L1 Adipocytes.Int J Mol Sci. 2020 Jan 10;21(2):460. doi: 10.3390/ijms21020460. Int J Mol Sci. 2020. PMID: 31936890 Free PMC article.
-
In Vitro Inhibitory Activity of Corilagin and Punicalagin Against Toxoplasma gondii and Their Mechanism(s) of Action.Antibiotics (Basel). 2025 Mar 24;14(4):336. doi: 10.3390/antibiotics14040336. Antibiotics (Basel). 2025. PMID: 40298470 Free PMC article.
References
-
- Sundar S., Rai M., Chakravarty J., Agarwal D., Agrawal N., Vaillant M., Olliaro P., Murray H.W. New treatment approach in Indian visceral leishmaniasis: Single-dose liposomal amphotericin B followed by short-course oral miltefosine. Clin. Infect. Dis. 2008;47:1000–1006. doi: 10.1086/591972. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources