Recombinant Expression of a Modified Shrimp Anti-Lipopolysaccharide Factor Gene in Pichia pastoris GS115 and Its Characteristic Analysis
- PMID: 27517939
- PMCID: PMC4999913
- DOI: 10.3390/md14080152
Recombinant Expression of a Modified Shrimp Anti-Lipopolysaccharide Factor Gene in Pichia pastoris GS115 and Its Characteristic Analysis
Abstract
Anti-lipopolysaccharide factors (ALFs) with a LPS-binding domain (LBD) are considered to have broad spectrum antimicrobial activities and certain antiviral properties in crustaceans. FcALF2 was one isoform of ALFs isolated from the Chinese shrimp Fenneropenaeus chinensis. Our previous study showed that a modified LBD domain (named LBDv) of FcALF2 exhibited a highly enhanced antimicrobial activity. In the present study, a modified FcALF2 gene (mFcALF2), in which the LBD was substituted by LBDv, was designed and synthesized. This gene was successfully expressed in yeast Pichia pastoris GS115 eukaryotic expression system, and the characteristics of the recombinant protein mFcALF2 were analyzed. mFcALF2 exhibited apparent antibacterial activities against Gram-negative bacteria, including Escherichia coli, Vibrio alginolyticus, Vibrio harveyi, and Vibrio parahaemolyticus, and Gram-positive bacteria, including Bacillus licheniformis and Staphylococcus epidermidis. In addition, mFcALF2 could reduce the propagation of white spot syndrome virus (WSSV) in vivo by pre-incubation with virus. The present study paves the way for developing antimicrobial drugs in aquaculture.
Keywords: anti-lipopolysaccharide factors; antibacterial activity; antiviral activity; recombinant protein.
Figures

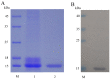





Similar articles
-
Recombinant expression and functional analysis of an isoform of anti-lipopolysaccharide factors (FcALF5) from Chinese shrimp Fenneropenaeus chinensis.Dev Comp Immunol. 2015 Nov;53(1):47-54. doi: 10.1016/j.dci.2015.06.015. Epub 2015 Jun 26. Dev Comp Immunol. 2015. PMID: 26123888
-
Characterization and function analysis of an anti-lipopolysaccharide factor (ALF) from the Chinese shrimp Fenneropenaeus chinensis.Dev Comp Immunol. 2014 Oct;46(2):349-55. doi: 10.1016/j.dci.2014.05.013. Epub 2014 May 28. Dev Comp Immunol. 2014. PMID: 24879940
-
Functional diversity of anti-lipopolysaccharide factor isoforms in shrimp and their characters related to antiviral activity.Mar Drugs. 2015 Apr 27;13(5):2602-16. doi: 10.3390/md13052602. Mar Drugs. 2015. PMID: 25923317 Free PMC article.
-
The Anti-lipopolysaccharide Factors in Crustaceans.Subcell Biochem. 2020;94:63-80. doi: 10.1007/978-3-030-41769-7_3. Subcell Biochem. 2020. PMID: 32189296 Review.
-
An intimate link between antimicrobial peptide sequence diversity and binding to essential components of bacterial membranes.Biochim Biophys Acta. 2016 May;1858(5):958-70. doi: 10.1016/j.bbamem.2015.10.011. Epub 2015 Oct 21. Biochim Biophys Acta. 2016. PMID: 26498397 Review.
Cited by
-
Silencing of suppressor of cytokine signaling 1 enhances the immunological effect of mucin 1-calreticulin-primed 4T1 cell-treated dendritic cells in breast cancer treatment.Oncol Lett. 2018 Feb;15(2):1630-1638. doi: 10.3892/ol.2017.7477. Epub 2017 Nov 23. Oncol Lett. 2018. PMID: 29434859 Free PMC article.
-
Evaluation of Therapeutic Efficiency of Stylicin against Vibrio parahaemolyticus Infection in Shrimp Penaeus vannamei through Comparative Proteomic Approach.Probiotics Antimicrob Proteins. 2024 Feb;16(1):76-92. doi: 10.1007/s12602-022-10006-w. Epub 2022 Dec 2. Probiotics Antimicrob Proteins. 2024. PMID: 36459385
-
Revolutionizing orthopedic healthcare: a systematic review unveiling recombinant antimicrobial peptides.Front Microbiol. 2024 Apr 29;15:1370826. doi: 10.3389/fmicb.2024.1370826. eCollection 2024. Front Microbiol. 2024. PMID: 38756724 Free PMC article.
-
The Transcriptomic Mechanism of a Novel Autolysis Induced by a Recombinant Antibacterial Peptide from Chicken Expressed in Pichia pastoris.Molecules. 2022 Mar 21;27(6):2029. doi: 10.3390/molecules27062029. Molecules. 2022. PMID: 35335392 Free PMC article.
-
Comparing the Ability of Secretory Signal Peptides for Heterologous Expression of Anti-Lipopolysaccharide Factor 3 in Chlamydomonas reinhardtii.Mar Drugs. 2023 Jun 4;21(6):346. doi: 10.3390/md21060346. Mar Drugs. 2023. PMID: 37367671 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

