Assay systems for screening food and natural substances that have anti-hyperuricemic activity: uric acid production in cultured hepatocytes and purine bodies-induced hyperuricemic model mice
- PMID: 27518104
- PMCID: PMC5461235
- DOI: 10.1007/s10616-016-0005-z
Assay systems for screening food and natural substances that have anti-hyperuricemic activity: uric acid production in cultured hepatocytes and purine bodies-induced hyperuricemic model mice
Abstract
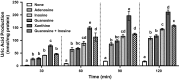
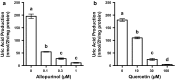
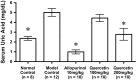
Hyperuricemia is characterized by the high uric acid (UA) level in serum (or plasma) and has been considered to be an important risk factor for gout. In the present study, we have attempted to construct an assay system for UA production in vitro employing cultured AML12 hepatocytes. UA levels in balanced salt solution (BSS) in the presence of UA precursor nucleosides, adenosine, inosine, guanosine and xanthine, at 12.5, 25, and 100 µM were significantly higher than BSS alone and their effects were dose-dependent, while all the UA precursors did not significantly increase intracellular UA levels. Hence, UA levels in BSS were thereafter adopted as an index of UA production. UA production from nucleosides was significantly higher than that from nucleotides (GMP, IMP and AMP). UA production from guanosine and inosine in combination (GI mixture) as well as nucleosides increased time-dependently and almost linearly up to 2 h. Selecting GI mixture, effects of allopurinol, a widely used anti-hyperuricemic agent, and quercetin, a well-known polyphenol in onion and strawberry, on UA production were examined. Both allopurinol and quercetin dose-dependently (0.1, 0.3 and 1 μM for allopurinol and 10, 30, and 100 μM for quercetin) and significantly reduced UA production in the hepatocytes. They also significantly reduced hyperuricemia induced by intraperitoneal injection of UA precursor purine bodies to mice at a single oral dose of 10 (allopurinol) or 200 (quercetin) mg/kg body weight. This assay system for UA production in cultured hepatocytes is considered to be useful to search for novel anti-hyperuricemic compounds in foods and natural resources with possibility to have human health benefits.
Keywords: AML12 hepatocyte; Hyperuricemia; Purine body; Uric acid.
Conflict of interest statement
Ethical Approval
This study was conducted on the basis of general ethical norms indicated by the Japan Society for the Promotion of Science (JSPS).
Conflict of interest
Authors declare that they have no conflict of interest.
Figures

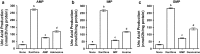
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

