Consequences of a Maternal High-Fat Diet and Late Gestation Diabetes on the Developing Rat Lung
- PMID: 27518105
- PMCID: PMC4982689
- DOI: 10.1371/journal.pone.0160818
Consequences of a Maternal High-Fat Diet and Late Gestation Diabetes on the Developing Rat Lung
Abstract
Rationale: Infants born to diabetic or obese mothers are at risk of respiratory distress and persistent pulmonary hypertension of the newborn (PPHN), conceivably through fuel-mediated pathogenic mechanisms. Prior research and preventative measures focus on controlling maternal hyperglycemia, but growing evidence suggests a role for additional circulating fuels including lipids. Little is known about the individual or additive effects of a maternal high-fat diet on fetal lung development.
Objective: The objective of this study was to determine the effects of a maternal high-fat diet, alone and alongside late-gestation diabetes, on lung alveologenesis and vasculogenesis, as well as to ascertain if consequences persist beyond the perinatal period.
Methods: A rat model was used to study lung development in offspring from control, diabetes-exposed, high-fat diet-exposed and combination-exposed pregnancies via morphometric, histologic (alveolarization and vasculogenesis) and physiologic (echocardiography, pulmonary function) analyses at birth and 3 weeks of age. Outcomes were interrogated for diet, diabetes and interaction effect using ANOVA with significance set at p≤0.05. Findings prompted additional mechanistic inquiry of key molecular pathways.
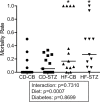
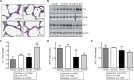
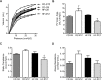
Results: Offspring exposed to maternal diabetes or high-fat diet, alone and in combination, had smaller lungs and larger hearts at birth. High-fat diet-exposed, but not diabetes-exposed offspring, had a higher perinatal death rate and echocardiographic evidence of PPHN at birth. Alveolar mean linear intercept, septal thickness, and airspace area (D2) were not significantly different between the groups; however, markers of lung maturity were. Both diabetes-exposed and diet-exposed offspring expressed more T1α protein, a marker of type I cells. Diet-exposed newborn pups expressed less surfactant protein B and had fewer pulmonary vessels enumerated. Mechanistic inquiry revealed alterations in AKT activation, higher endothelin-1 expression, and an impaired Txnip/VEGF pathway that are important for vessel growth and migration. After 3 weeks, mortality remained highest and static lung compliance and hysteresis were lowest in combination-exposed offspring.
Conclusion: This study emphasizes the effects of a maternal high-fat diet, especially alongside late-gestation diabetes, on pulmonary vasculogenesis, demonstrates adverse consequences beyond the perinatal period and directs attention to mechanistic pathways of interest. Findings provide a foundation for additional investigation of preventative and therapeutic strategies aimed at decreasing pulmonary morbidity in at-risk infants.
Conflict of interest statement
Figures


References
-
- Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes care. 2005;28(3):579–84. Epub 2005/03/01. . - PubMed
-
- Dennedy MC, Avalos G, O'Reilly MW, O'Sullivan EP, Dunne FP. The impact of maternal obesity on gestational outcomes. Ir Med J. 2012;105(5 Suppl):23–5. Epub 2012/07/31. . - PubMed
-
- Sutton L, Sayer GP, Bajuk B, Richardson V, Berry G, Henderson-Smart DJ. Do very sick neonates born at term have antenatal risks? 2. Infants ventilated primarily for lung disease. Acta obstetricia et gynecologica Scandinavica. 2001;80(10):917–25. Epub 2001/10/03. . - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

