Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children
- PMID: 27518660
- PMCID: PMC5137793
- DOI: 10.1056/NEJMoa1508749
Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children
Abstract
Background: The Amish and Hutterites are U.S. agricultural populations whose lifestyles are remarkably similar in many respects but whose farming practices, in particular, are distinct; the former follow traditional farming practices whereas the latter use industrialized farming practices. The populations also show striking disparities in the prevalence of asthma, and little is known about the immune responses underlying these disparities.
Methods: We studied environmental exposures, genetic ancestry, and immune profiles among 60 Amish and Hutterite children, measuring levels of allergens and endotoxins and assessing the microbiome composition of indoor dust samples. Whole blood was collected to measure serum IgE levels, cytokine responses, and gene expression, and peripheral-blood leukocytes were phenotyped with flow cytometry. The effects of dust extracts obtained from Amish and Hutterite homes on immune and airway responses were assessed in a murine model of experimental allergic asthma.
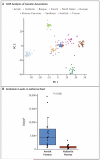
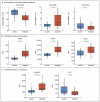
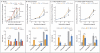
Results: Despite the similar genetic ancestries and lifestyles of Amish and Hutterite children, the prevalence of asthma and allergic sensitization was 4 and 6 times as low in the Amish, whereas median endotoxin levels in Amish house dust was 6.8 times as high. Differences in microbial composition were also observed in dust samples from Amish and Hutterite homes. Profound differences in the proportions, phenotypes, and functions of innate immune cells were also found between the two groups of children. In a mouse model of experimental allergic asthma, the intranasal instillation of dust extracts from Amish but not Hutterite homes significantly inhibited airway hyperreactivity and eosinophilia. These protective effects were abrogated in mice that were deficient in MyD88 and Trif, molecules that are critical in innate immune signaling.
Conclusions: The results of our studies in humans and mice indicate that the Amish environment provides protection against asthma by engaging and shaping the innate immune response. (Funded by the National Institutes of Health and others.).
Figures

Comment in
-
Innate Immunity in Asthma.N Engl J Med. 2016 Aug 4;375(5):477-9. doi: 10.1056/NEJMe1607438. N Engl J Med. 2016. PMID: 27518667 Free PMC article. No abstract available.
-
Asthma and allergy: Old McDonald had a farm (but no asthma)!Nat Rev Immunol. 2016 Aug 25;16(9):535. doi: 10.1038/nri.2016.97. Nat Rev Immunol. 2016. PMID: 27558411 No abstract available.
-
Innate Immunity and Asthma Risk.N Engl J Med. 2016 Nov 10;375(19):1898-1899. doi: 10.1056/NEJMc1611699. N Engl J Med. 2016. PMID: 27959651 Free PMC article. No abstract available.
-
Innate Immunity and Asthma Risk.N Engl J Med. 2016 Nov 10;375(19):1897. doi: 10.1056/NEJMc1611699. N Engl J Med. 2016. PMID: 27959652 No abstract available.
-
Innate Immunity and Asthma Risk.N Engl J Med. 2016 Nov 10;375(19):1897. doi: 10.1056/NEJMc1611699. N Engl J Med. 2016. PMID: 28112884 No abstract available.
-
Innate Immunity and Asthma Risk.N Engl J Med. 2016 Nov 10;375(19):1897-1898. doi: 10.1056/NEJMc1611699. N Engl J Med. 2016. PMID: 28112885 No abstract available.
-
Insights into Asthma Therapies, Cardiovascular Effects, and Mechanisms from Recent Clinical Trials.Am J Respir Crit Care Med. 2017 Oct 1;196(7):920-922. doi: 10.1164/rccm.201702-0428RR. Am J Respir Crit Care Med. 2017. PMID: 28812907 No abstract available.
References
-
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. - PubMed
-
- Braun-Fahrländer C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. - PubMed
-
- Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
