Hyperglycemia Determines Increased Specific MicroRNAs Levels in Sera and HDL of Acute Coronary Syndrome Patients and Stimulates MicroRNAs Production in Human Macrophages
- PMID: 27519051
- PMCID: PMC4982674
- DOI: 10.1371/journal.pone.0161201
Hyperglycemia Determines Increased Specific MicroRNAs Levels in Sera and HDL of Acute Coronary Syndrome Patients and Stimulates MicroRNAs Production in Human Macrophages
Abstract
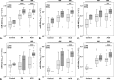
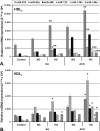
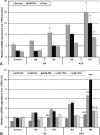
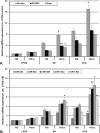
We aimed to determine the levels of microRNAs (miRNAs) in sera and HDL of acute coronary syndrome (ACS) compared to stable angina (SA) patients with/without hyperglycemia, and evaluate comparatively the functional effect of these sera on the processing machinery proteins (Drosha, DGCR8, Dicer) and miRNAs production in human macrophages. MiRNAs levels in sera and HDL from 35 SA and 72 ACS patients and 30 healthy subjects were measured by using microRNA TaqMan assays. MiR-223, miR-92a, miR-486, miR-122, miR-125a and miR-146a levels were higher in the hyperglycemic ACS compared to normoglycemic sera. MiR-223 and miR-486 prevailed in HDL2, while miR-92a predominated in HDL3, all three miRNAs discriminating between ACS and SA patients; their levels were increased in HDL from hyperglycemic ACS patients versus normoglycemic ones. The incubation of human macrophages with sera from ACS and SA patients showed that all patients' sera induced an increase of Drosha, DGCR8 and Dicer expressions and of selected miRNAs levels compared to control sera, the effect being higher in the case of hyperglycemic versus normoglycemic ACS sera. The addition of glucose to SA and ACS sera increased Drosha, DGCR8 and Dicer expression and miRNAs levels in the exposed macrophages. In conclusion, hyperglycemia is associated with increased miR-223, miR-92a, miR-486 levels in HDL, which discriminate between ACS and SA patients. Exposure of human macrophages to ACS compared to SA sera determines the upregulation of Drosha, DGCR8 and Dicer expression and the increase of selected miRNAs production, the effect being augmented by an increased glucose concentration.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

