Extracellular Polyphosphate Inhibits Proliferation in an Autocrine Negative Feedback Loop in Dictyostelium discoideum
- PMID: 27519410
- PMCID: PMC5025707
- DOI: 10.1074/jbc.M116.737825
Extracellular Polyphosphate Inhibits Proliferation in an Autocrine Negative Feedback Loop in Dictyostelium discoideum
Abstract
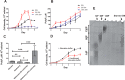
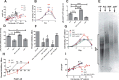
Polyphosphate is a polymer of phosphate residues linked by high energy phosphoanhydride bonds. Despite being highly conserved throughout nature, its function is poorly understood. Here we show that Dictyostelium cells accumulate extracellular polyphosphate, and this acts to inhibit proliferation at high cell densities. In shaking culture, extracellular polyphosphate concentrations increase as cell density increases, and if the concentration of polyphosphate observed at the stationary phase is added to cells at mid-log, proliferation is halted. Adding an exopolyphosphatase to cell cultures or stationary phase conditioned medium decreases polyphosphate levels and abrogates the anti-proliferative effect. The cells show saturable binding of polyphosphate, suggesting the presence of a cell surface polyphosphate receptor. Extracellular polyphosphate accumulation is potentiated by decreased nutrient levels, potentially as a means to anticipate starvation. Loss of the Dictyostelium polyphosphate kinase DdPpk1 causes intracellular polyphosphate levels to become undetectable and negatively affects fitness, cytokinesis, and germination. However, cells lacking DdPpk1 accumulate ∼50% normal levels of extracellular polyphosphate, suggesting an additional means of synthesis. We found that cells lacking inositol hexakisphosphate kinase, which is responsible for the synthesis of the inositol pyrophosphates IP7 and IP8, reach abnormally high cell densities and show decreased extracellular polyphosphate levels. Two different enzymes thus appear to mediate the synthesis of Dictyostelium extracellular polyphosphate, which is used as a signal in an autocrine negative feedback loop to regulate cell proliferation.
Keywords: Dictyostelium; cell biology; cell growth; cell proliferation; inositol hexakisphosphate kinase; polyphosphate; polyphosphate kinase; stress.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
The inositol pyrophosphate metabolism of Dictyostelium discoideum does not regulate inorganic polyphosphate (polyP) synthesis.Adv Biol Regul. 2022 Jan;83:100835. doi: 10.1016/j.jbior.2021.100835. Epub 2021 Nov 10. Adv Biol Regul. 2022. PMID: 34782304 Free PMC article.
-
Developmental accumulation of inorganic polyphosphate affects germination and energetic metabolism in Dictyostelium discoideum.Proc Natl Acad Sci U S A. 2016 Jan 26;113(4):996-1001. doi: 10.1073/pnas.1519440113. Epub 2016 Jan 11. Proc Natl Acad Sci U S A. 2016. PMID: 26755590 Free PMC article.
-
The putative G protein-coupled receptor GrlD mediates extracellular polyphosphate sensing in Dictyostelium discoideum.Mol Biol Cell. 2019 Apr 15;30(9):1118-1128. doi: 10.1091/mbc.E18-10-0686. Epub 2019 Feb 20. Mol Biol Cell. 2019. PMID: 30785840 Free PMC article.
-
Inorganic polyphosphate in the origin and survival of species.Proc Natl Acad Sci U S A. 2004 Nov 16;101(46):16085-7. doi: 10.1073/pnas.0406909101. Epub 2004 Nov 1. Proc Natl Acad Sci U S A. 2004. PMID: 15520374 Free PMC article. Review.
-
[Progress in polyphosphate and related metabolizing enzymes].Sheng Li Ke Xue Jin Zhan. 2011 Jun;42(3):181-7. Sheng Li Ke Xue Jin Zhan. 2011. PMID: 21932515 Review. Chinese.
Cited by
-
Inorganic polyphosphates stimulates matrix production in human annulus fibrosus cells.JOR Spine. 2021 Mar 2;4(2):e1143. doi: 10.1002/jsp2.1143. eCollection 2021 Jun. JOR Spine. 2021. PMID: 34337332 Free PMC article.
-
Stabilization of Murine Norovirus by Bacteria.mSphere. 2022 Jun 29;7(3):e0004622. doi: 10.1128/msphere.00046-22. Epub 2022 May 9. mSphere. 2022. PMID: 35531660 Free PMC article.
-
Controlling organism size by regulating constituent cell numbers.Proc IEEE Conf Decis Control. 2018 Dec;2018:2685-2690. doi: 10.1109/CDC.2018.8619546. Epub 2019 Jan 21. Proc IEEE Conf Decis Control. 2018. PMID: 30886453 Free PMC article.
-
Polyphosphate as a Target for Interference With Inflammation and Thrombosis.Front Med (Lausanne). 2019 Apr 12;6:76. doi: 10.3389/fmed.2019.00076. eCollection 2019. Front Med (Lausanne). 2019. PMID: 31106204 Free PMC article. Review.
-
Inorganic Polyphosphates As Storage for and Generator of Metabolic Energy in the Extracellular Matrix.Chem Rev. 2019 Dec 26;119(24):12337-12374. doi: 10.1021/acs.chemrev.9b00460. Epub 2019 Nov 18. Chem Rev. 2019. PMID: 31738523 Free PMC article. Review.
References
-
- Rao N. N., Gómez-García M. R., and Kornberg A. (2009) Inorganic polyphosphate: essential for growth and survival. Annu. Rev. Biochem. 78, 605–647 - PubMed
-
- Kuroda A., Nomura K., Ohtomo R., Kato J., Ikeda T., Takiguchi N., Ohtake H., and Kornberg A. (2001) Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 293, 705–708 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

