Architecture of the Human Mitochondrial Iron-Sulfur Cluster Assembly Machinery
- PMID: 27519411
- PMCID: PMC5076535
- DOI: 10.1074/jbc.M116.738542
Architecture of the Human Mitochondrial Iron-Sulfur Cluster Assembly Machinery
Abstract
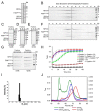
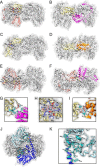
Fe-S clusters, essential cofactors needed for the activity of many different enzymes, are assembled by conserved protein machineries inside bacteria and mitochondria. As the architecture of the human machinery remains undefined, we co-expressed in Escherichia coli the following four proteins involved in the initial step of Fe-S cluster synthesis: FXN42-210 (iron donor); [NFS1]·[ISD11] (sulfur donor); and ISCU (scaffold upon which new clusters are assembled). We purified a stable, active complex consisting of all four proteins with 1:1:1:1 stoichiometry. Using negative staining transmission EM and single particle analysis, we obtained a three-dimensional model of the complex with ∼14 Å resolution. Molecular dynamics flexible fitting of protein structures docked into the EM map of the model revealed a [FXN42-210]24·[NFS1]24·[ISD11]24·[ISCU]24 complex, consistent with the measured 1:1:1:1 stoichiometry of its four components. The complex structure fulfills distance constraints obtained from chemical cross-linking of the complex at multiple recurring interfaces, involving hydrogen bonds, salt bridges, or hydrophobic interactions between conserved residues. The complex consists of a central roughly cubic [FXN42-210]24·[ISCU]24 sub-complex with one symmetric ISCU trimer bound on top of one symmetric FXN42-210 trimer at each of its eight vertices. Binding of 12 [NFS1]2·[ISD11]2 sub-complexes to the surface results in a globular macromolecule with a diameter of ∼15 nm and creates 24 Fe-S cluster assembly centers. The organization of each center recapitulates a previously proposed conserved mechanism for sulfur donation from NFS1 to ISCU and reveals, for the first time, a path for iron donation from FXN42-210 to ISCU.
Keywords: Friedreich ataxia; frataxin; iron-sulfur protein; mitochondria; protein complex.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures












Similar articles
-
Structure and functional dynamics of the mitochondrial Fe/S cluster synthesis complex.Nat Commun. 2017 Nov 3;8(1):1287. doi: 10.1038/s41467-017-01497-1. Nat Commun. 2017. PMID: 29097656 Free PMC article.
-
Defining the Architecture of the Core Machinery for the Assembly of Fe-S Clusters in Human Mitochondria.Methods Enzymol. 2017;595:107-160. doi: 10.1016/bs.mie.2017.07.003. Epub 2017 Aug 18. Methods Enzymol. 2017. PMID: 28882199
-
The N-Terminus of Iron-Sulfur Cluster Assembly Factor ISD11 Is Crucial for Subcellular Targeting and Interaction with l-Cysteine Desulfurase NFS1.Biochemistry. 2017 Mar 28;56(12):1797-1808. doi: 10.1021/acs.biochem.6b01239. Epub 2017 Mar 17. Biochemistry. 2017. PMID: 28271877
-
Molecular Details of the Frataxin-Scaffold Interaction during Mitochondrial Fe-S Cluster Assembly.Int J Mol Sci. 2021 Jun 2;22(11):6006. doi: 10.3390/ijms22116006. Int J Mol Sci. 2021. PMID: 34199378 Free PMC article. Review.
-
Mechanisms of Mitochondrial Iron-Sulfur Protein Biogenesis.Annu Rev Biochem. 2020 Jun 20;89:471-499. doi: 10.1146/annurev-biochem-013118-111540. Epub 2020 Jan 14. Annu Rev Biochem. 2020. PMID: 31935115 Review.
Cited by
-
Iron-sulfur cluster biogenesis and trafficking in mitochondria.J Biol Chem. 2017 Aug 4;292(31):12754-12763. doi: 10.1074/jbc.R117.787101. Epub 2017 Jun 14. J Biol Chem. 2017. PMID: 28615445 Free PMC article. Review.
-
SAXS and stability studies of iron-induced oligomers of bacterial frataxin CyaY.PLoS One. 2017 Sep 20;12(9):e0184961. doi: 10.1371/journal.pone.0184961. eCollection 2017. PLoS One. 2017. PMID: 28931050 Free PMC article.
-
Biallelic mutations in the ferredoxin reductase gene cause novel mitochondriopathy with optic atrophy.Hum Mol Genet. 2017 Dec 15;26(24):4937-4950. doi: 10.1093/hmg/ddx377. Hum Mol Genet. 2017. PMID: 29040572 Free PMC article.
-
Characterization of [2Fe-2S]-Cluster-Bridged Protein Complexes and Reaction Intermediates by use of Native Mass Spectrometric Methods.Angew Chem Int Ed Engl. 2020 Apr 20;59(17):6724-6728. doi: 10.1002/anie.201915615. Epub 2020 Mar 3. Angew Chem Int Ed Engl. 2020. PMID: 32031732 Free PMC article.
-
Biophysical characterisation of the recombinant human frataxin precursor.FEBS Open Bio. 2018 Jan 25;8(3):390-405. doi: 10.1002/2211-5463.12376. eCollection 2018 Mar. FEBS Open Bio. 2018. PMID: 29511616 Free PMC article.
References
-
- Lill R., Hoffmann B., Molik S., Pierik A. J., Rietzschel N., Stehling O., Uzarska M. A., Webert H., Wilbrecht C., and Mühlenhoff U. (2012) The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta 1823, 1491–1508 - PubMed
-
- Blanc B., Gerez C., and Ollagnier de Choudens S. (2015) Assembly of Fe/S proteins in bacterial systems: biochemistry of the bacterial ISC system. Biochim. Biophys. Acta 1853, 1436–1447 - PubMed
-
- Layer G., Ollagnier-de Choudens S., Sanakis Y., and Fontecave M. (2006) Iron-sulfur cluster biosynthesis: characterization of Escherichia coli CyaY as an iron donor for the assembly of [2Fe-2S] clusters in the scaffold IscU. J. Biol. Chem. 281, 16256–16263 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

